22 October 2024
By Maynard Paton
H1 2024 results summary for Bioventix (BVXP):
- A record H1, showing revenue up 13%, profit up 15% and the dividend up 10% as greater sales to China helped offset a “temporary” slowing of troponin growth.
- Positive long-term progress continues to rest upon Alzheimer’s R&D, in which a study involving pTau212 suggests BVXP may enjoy a research edge beyond an increasingly crowded pTau217 field.
- BVXP’s Alzheimer’s work on ‘brain-derived tau’ has attracted “significant attention” for helping identify significant, near-term “cognitive decline”, and is now available through Quanterix for wider research purposes.
- The economics of successful antibodies remain superb, with this H1 displaying a terrific 77% operating margin, net cash equivalent to a hefty 40% of revenue and capex of just £5k.
- The £37 shares yield a trailing 4.3%, trade at a possible 22x P/E and might — using some very basic assumptions — value the R&D pipeline at £23m. I continue to hold.
Contents
- News links, share data and disclosure
- Why I own BVXP
- Results summary
- Revenue, profit and dividend
- Royalty income and physical sales
- Vitamin D
- Troponin and other antibodies
- Pipeline: Alzheimer’s and pTau217
- Pipeline: Alzheimer’s and brain-derived tau
- Pipeline: biomonitoring
- Pipeline: CardiNor and Pre-Diagnostics
- Financials: margin and employees
- Financials: cash flow and balance sheet
- Valuation
News links, share data and disclosure
- Interim results and presentation for the six months to 31 December 2023 published 25 March 2024.
- Share price: £37
- Share count: 5,219,656
- Market capitalisation: £193m
- Disclosure: Maynard owns shares in Bioventix. This blog post contains SharePad affiliate links.
Why I own BVXP

- Develops diagnostic blood-test antibodies, direct competition for which is limited due to the necessary scientific innovation, protracted regulatory testing, onerous switching procedures and ‘captive’ hospital end-customers.
- Boasts founder/entrepreneurial chief exec who has overseen an attractive growth record, prefers a cash-rich balance sheet, retains a 6%/£11m shareholding and has declared seven special dividends.
- Employs ‘scalable’ royalty/licensing model that requires few employees and leads to terrific margins, generous cash flow and immense returns on retained profits.
Further reading: My BVXP Buy report | All my BVXP posts | BVXP website
Results summary
Revenue, profit and dividend
- The preceding FY’s outlook referring only to the long term…
[FY 2023] “We are pleased with our financial results for the year which we believe reflect steady growth in the use of our established products and the continued roll-out of the high sensitivity troponin assays.
Excellent technical progress has been made with our research projects and we anticipate that our pipeline of opportunities will create additional shareholder value in the later 2020s and 2030s andwe look forward to further progress in the years ahead.“
- …had left the outcome of this H1 somewhat of a mystery.
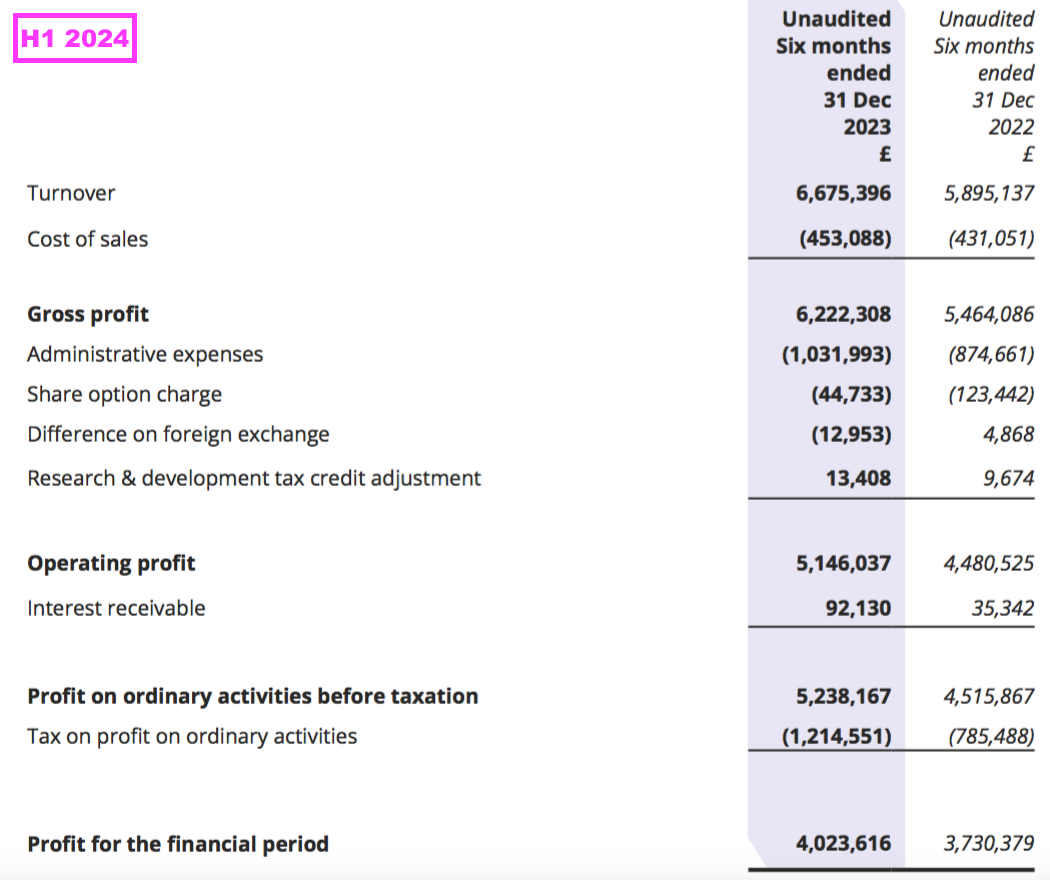
- In the event, H1 revenue gained 13% to £6.7m and set a new H1 record:
- The 13% improvement was ahead of the “sustainable” 8-10% growth rate cited during FY 2022…
[FY 2022] “The growth in our underlying business over the year is in the range 8-10% which we believe is sustainable for the immediate future as our sales mix continues to change.”
- ...and did not appear to be bolstered by currency movements.
- The preceding FY reiterated at least 50% of revenue is earned in USD…
[FY 2023] “We estimate that between 50% and 60% of our total revenue is either directly linked to US Dollars via physical product pricing in Dollars or indirectly linked to US Dollars via royalties based on downstream Dollar sales. The remainder of the currency split is dominated by Euros and important Asian currencies. In the past year, exchange rates have been relatively stable compared to the previous 12 months.”
- …and FY 2021 explained how exchange rates influence revenue:
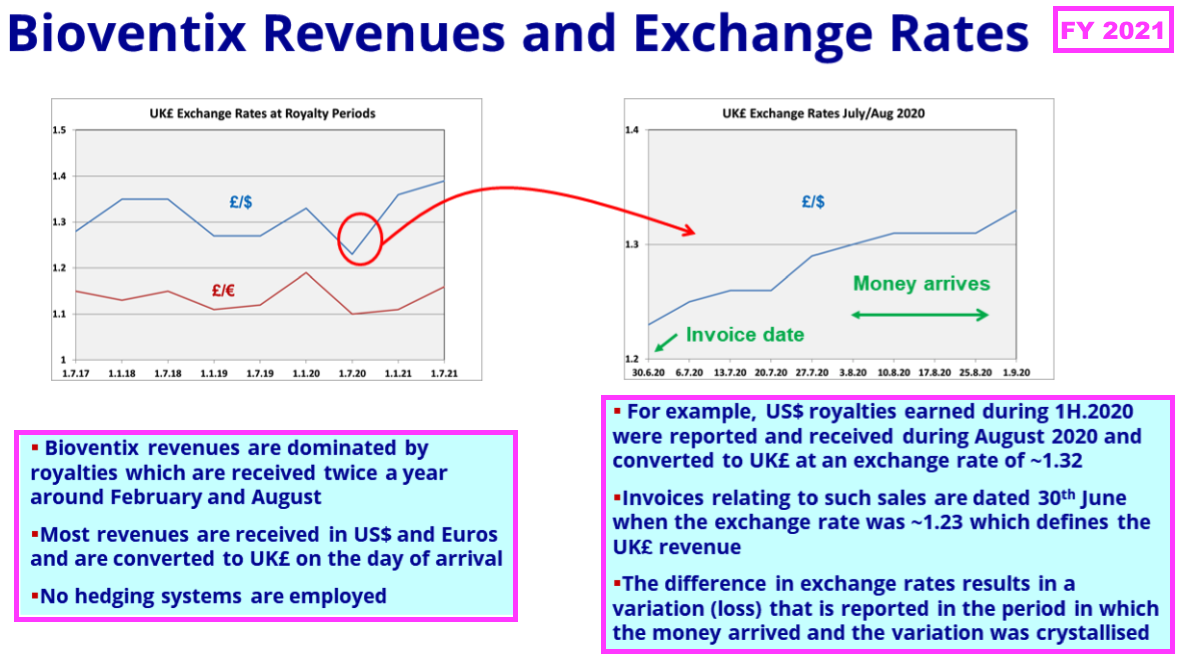
- Royalty invoices for this H1 were dated 31 December 2023, at which point GBP:USD was 1.275.
- Royalty invoices for the comparable H1 were dated 31 December 2022, at which point GBP:USD was 1.209.
- As such, USD-invoiced royalty revenue was translated into GBP at a 5% lower rate during this H1 versus the comparable H1.
- BVXP’s H1’s revenue progress looks to have exceeded that of Roche, the world’s largest in vitro diagnostic (IVD) company and as good a benchmark as any to judge demand for the type of blood-test antibodies BVXP develops.
- The comparable six months (July to December 2023) at Roche witnessed diagnostic revenue (excluding Covid-related income) decline 3% to CHF6.5b:
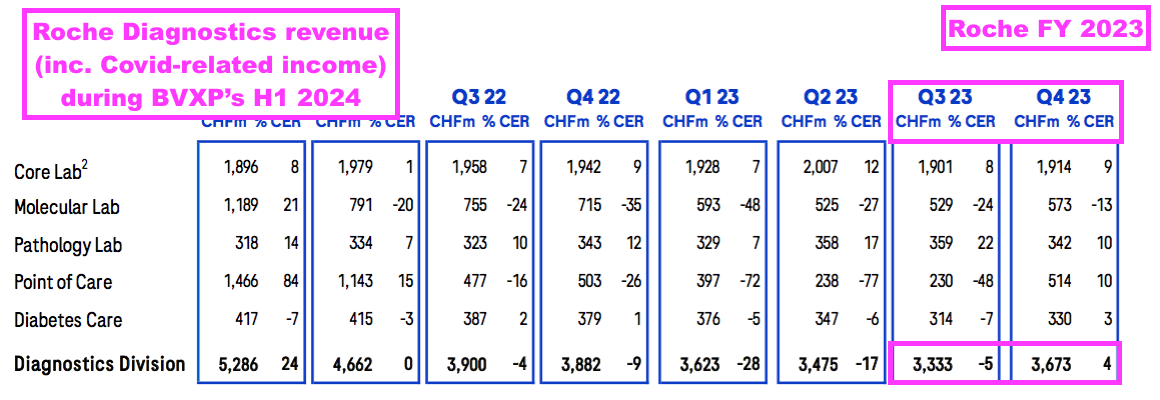
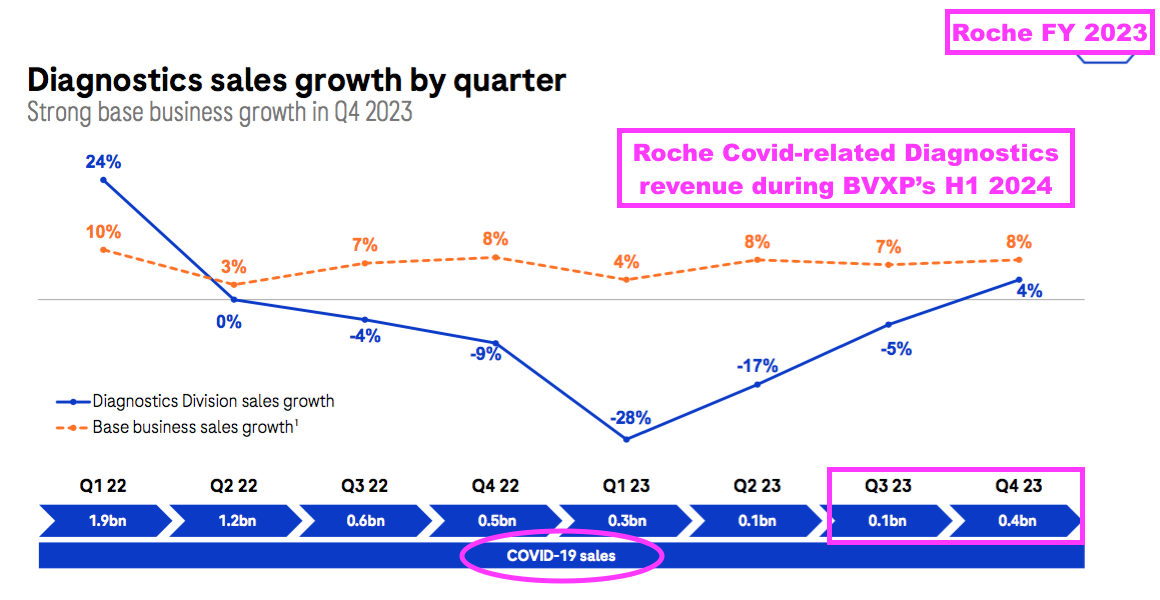
- BVXP appears to have outperformed Roche before, during and after the pandemic.
- I estimate Roche’s routine-diagnostic sales expanded 2% (from CHF12.9b to CHF13.4b) between calendar 2019 and calendar 2023:
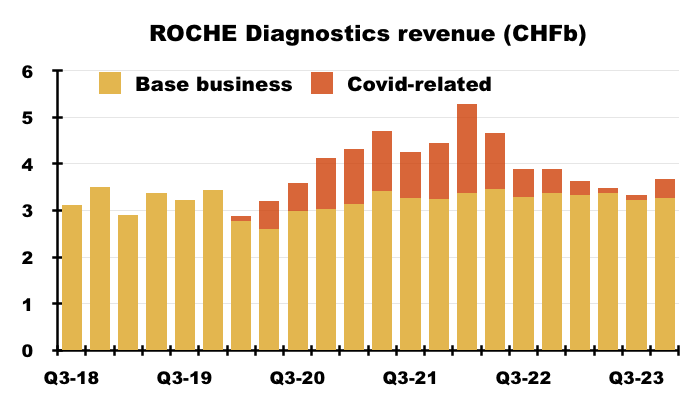
- BVXP’s revenue meanwhile advanced 36% (from £10.0m to £13.6m) during the same four years.
- The 13% H1 revenue gain pushed operating profit up 15% to £5.4m, which set a new H1 record:
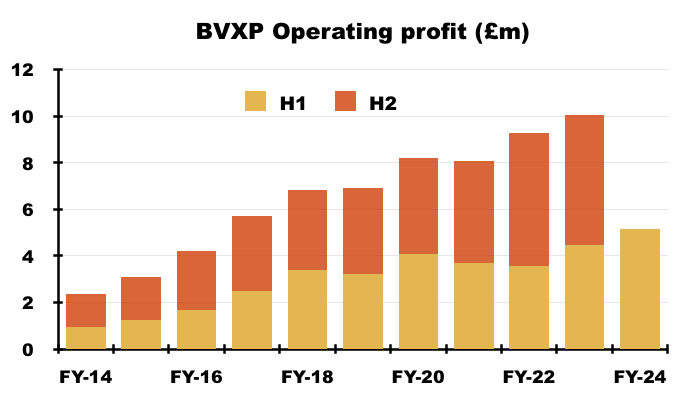
- The profit increase led to the H1 dividend being lifted 10% to 68p per share:
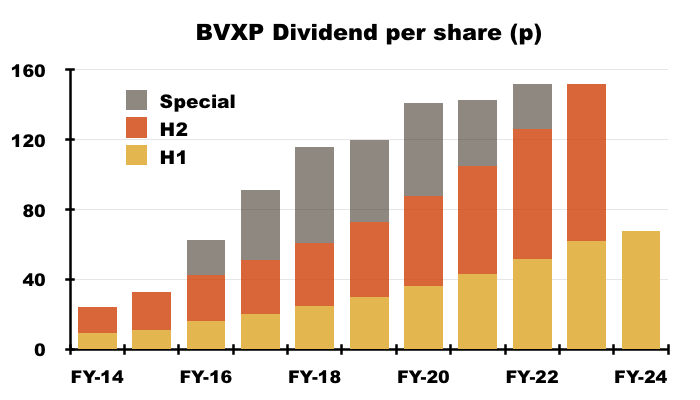
- The preceding FY ended a seven-year run of special annual dividends, and this H1 said future payouts would advance in tandem with earnings:
“Recent changes to the headline rate of Corporation Tax has had an impact on our reported earnings and cash flows. Nevertheless, we will endeavour to follow our established dividend policy of increasing our distributions to shareholders in line with increases in our post tax profit.”
- This H1 presented the house broker’s 173p per share dividend estimate for FY 2024:
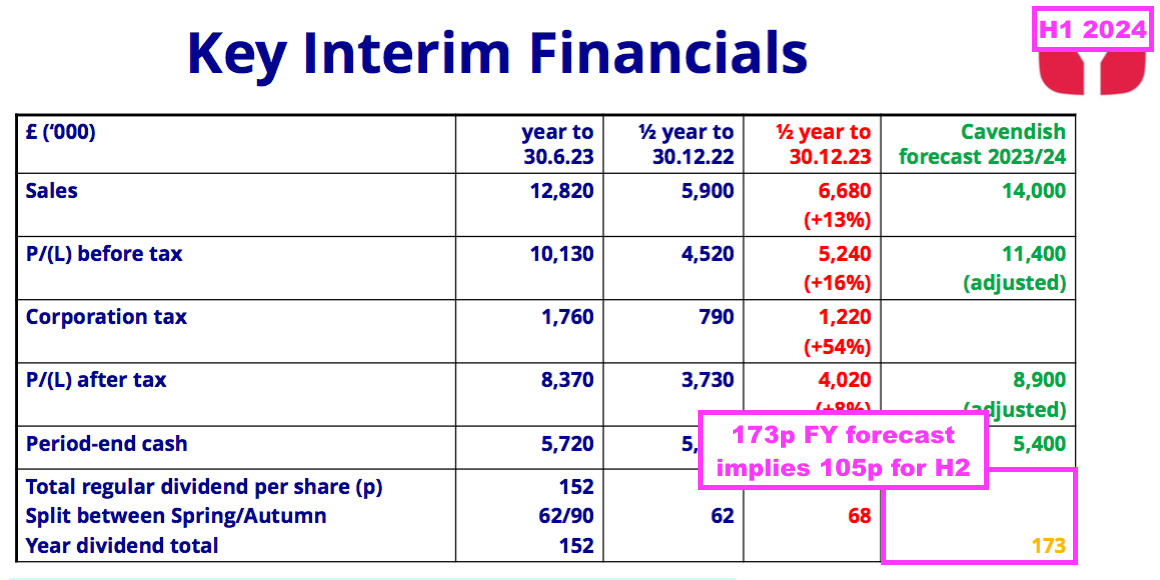
- A 173p per share projected FY payout implies 105p per share for H2 2024 — up a useful 17% on the 90p per share declared for H2 2023.
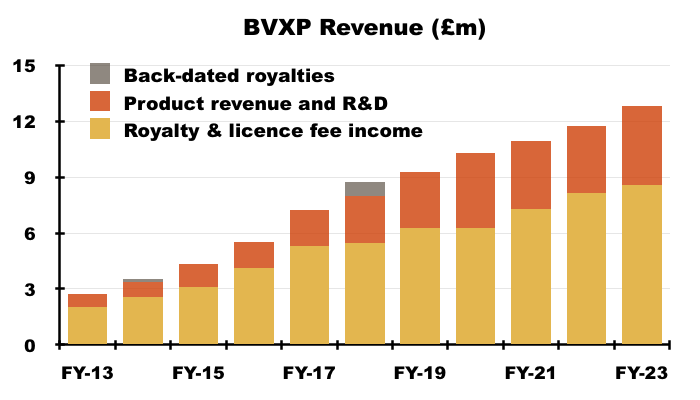
Royalty income and physical sales
- Royalties and licence fees have historically represented the bulk of BVXP’s revenue:
- Management explained during the 2022 AGM that customers can:
- Manufacture BVXP’s antibodies under licence and pay a royalty for their subsequent usage, and/or;
- Buy BVXP’s antibodies in physical form.
- Whether payments are made through royalties/licence fees or physical sales is determined mostly by the volume of antibodies required for a particular blood test. Some tests require 100s or 1000s more antibodies than others:
- Antibodies used in large volumes tend to be sold physically and collect only small royalties, whereas antibodies used in small volumes tend to have small physical sales but collect larger royalties.
- Royalty rates are typically around 2% of the price the end user (e.g. a hospital) pays BVXP’s customer (e.g. a blood-test machine manufacturer) for the blood-test reagent packs.
- BVXP traditionally divulges the split between royalty income and physical sales only within its FY results.
- This H1 simply suggested the 13% revenue uplift was biased towards physical sales to China:
“Sales of physical products have performed well and revenues from our vitamin D antibody and other core antibodies have all increased as anticipated. A significant element of the growth came from increased physical product sales and associated royalties from our Chinese customers.”
- China does appear to be a growing market for BVXP; the preceding FY also noted physical sales were helped by greater Chinese demand:
[FY 2023] “Our shipments of physical antibody to China continued to increase. Some sales are made directly but the majority are made through five appointed distributors. Regulatory approvals for domestic Chinese customers have considerable lead times but we continue to see modest increases in royalty payments owing from these customers.”
- Indeed, the preceding FY revealed China accounted for approximately half of all physical sales:
[FY 2023] “Sales to China remain strong accounting for ~50% of total physical sales. Chinese downstream assay price pressure has increased through aggressive central health authority procurement mechanisms”
- Mind you, the preceding FY also noted greater Chinese demand may lead to greater Chinese risks:
[FY 2023] “The prospects for further growth in China are good although we detect pressure on downstream final assay prices that has resulted from aggressive purchasing on the part of centralised procurement organisations. This could exert pressure on Bioventix as we supply relatively high-cost reagents. We also recognise that continued antibody technology development in China and elsewhere constitutes a longer-term threat.“
- BVXP’s reluctance to split H1 revenue between royalty income and physical sales may be due to the incredible economics of commercial antibodies (see Financials: margin and employees).
- In particular, the preceding FY reiterated the tiny volume of physical antibodies sold…
[FY 2023] “We currently sell a total of 15–20 grams of purified physical antibody per year“
- …versus revenue from physical sales of up to £4.2m, which implied antibodies may sell for up to £210k a gram.
- For perspective, gold sells for approximately $2,650 an ounce, which is equivalent to £65 a gram.
Vitamin D
- By far BVXP’s best seller is its vitamin D antibody, which has represented between 38% and 47% of revenue since FY 2016:
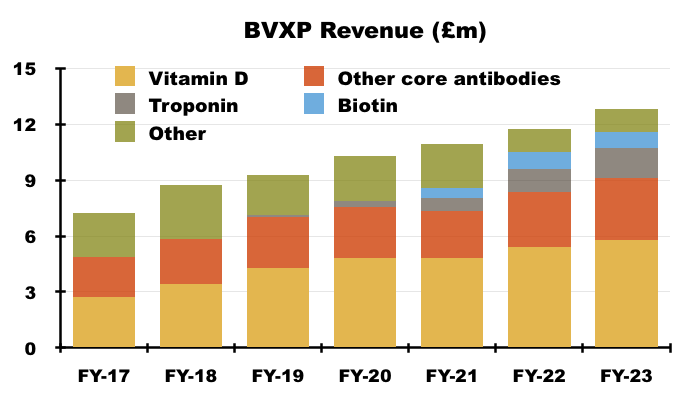
- Just like the split between royalty income and physical sales, BVXP only divulges the revenue from its leading antibodies within its FY results.
- H1 presentations therefore (oddly) repeat the slide showing the individual antibody contributions from the preceding FY:
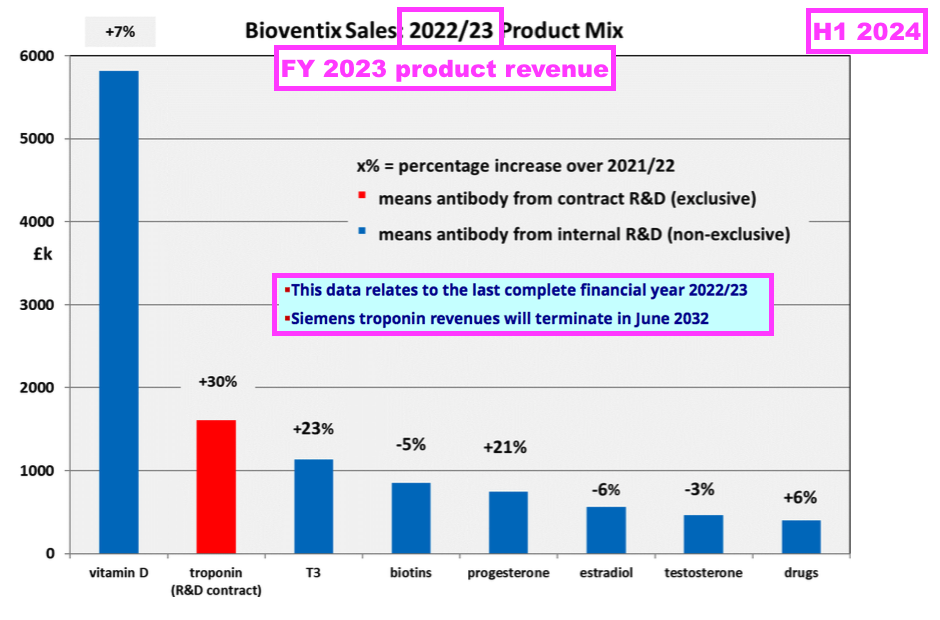
- I speculate BVXP’s reluctance to disclose H1 individual antibody revenue is due to the company’s apparent limited visibility towards physical sales and royalty income, which could leave six-month fluctuations open to misinterpretation by shareholders.
- This H1 provided only the shortest of updates on the vitamin D best-seller:
“Sales of physical products have performed well and revenues from our vitamin D antibody and other core antibodies have all increased as anticipated.”
- At least BVXP was consistent with all shareholders for this H1, and did not tip off its broker about individual H1 antibody revenue.
- The preceding FY revealed vitamin D revenue had gained 7% and implied vitamin D growth expectations were between 5% and 10%:
[FY 2023] “Vitamin D and core antibody sales were in line with an expectation of 5-10% annual growth“.
- If vitamin D revenue “increased as expected” during this H1, then perhaps that increase was a minimum 5% to take annual vitamin D revenue even closer to £6m.
- At least vitamin D revenue is still increasing. BVXP began claiming during FY 2017 that vitamin D sales would “plateau” and two years ago talked of “price erosion in downstream markets“:
[H1 2022] “As reported previously, the growth rates for our vitamin D antibody sales were not expected to match those seen in recent financial years and a plateau in the downstream global vitamin D assay market had been anticipated. Sales associated with assay formats using larger quantities of antibody per test suffered more as price erosion in downstream markets puts pressure on costly “antibody-hungry” products“.
- An informative broker note issued after this H1 included some very useful background to vitamin D antibodies.
- In particular, the broker calculated 36 vitamin D tests were approved for use within the United States between 2010 and 2023:
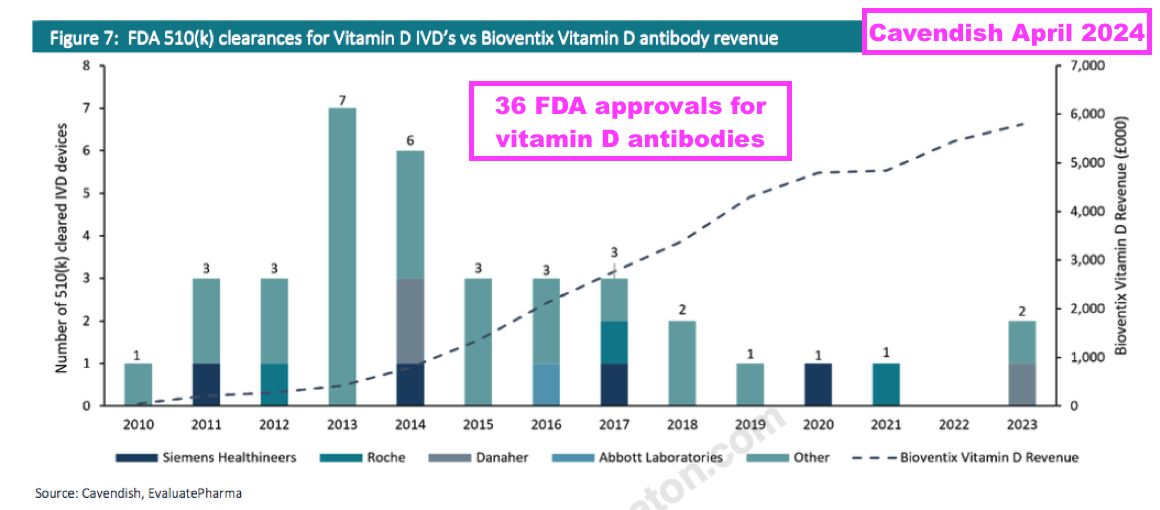
- In addition, the broker reckoned another nine vitamin D tests received US regulatory approval between 1995 and 2010.
- BVXP’s development on vitamin D started during 2008 and commercialisation began during 2011.
- The broker concluded BVXP’s vitamin D antibody delivered meaningful revenue over time despite:
- A growing number of alternatives, and;
- Not being first to market….
- …which may have favourable implications for any new BVXP antibody (see Pipeline: Alzheimer’s and pTau217 and Pipeline: Alzheimer’s and brain-derived tau).
- Commercial antibody longevity is due mainly to the healthcare/IVD industry’s reluctance to replace a proven blood test, unless the upgraded version offers significant diagnostic improvements over the incumbent:
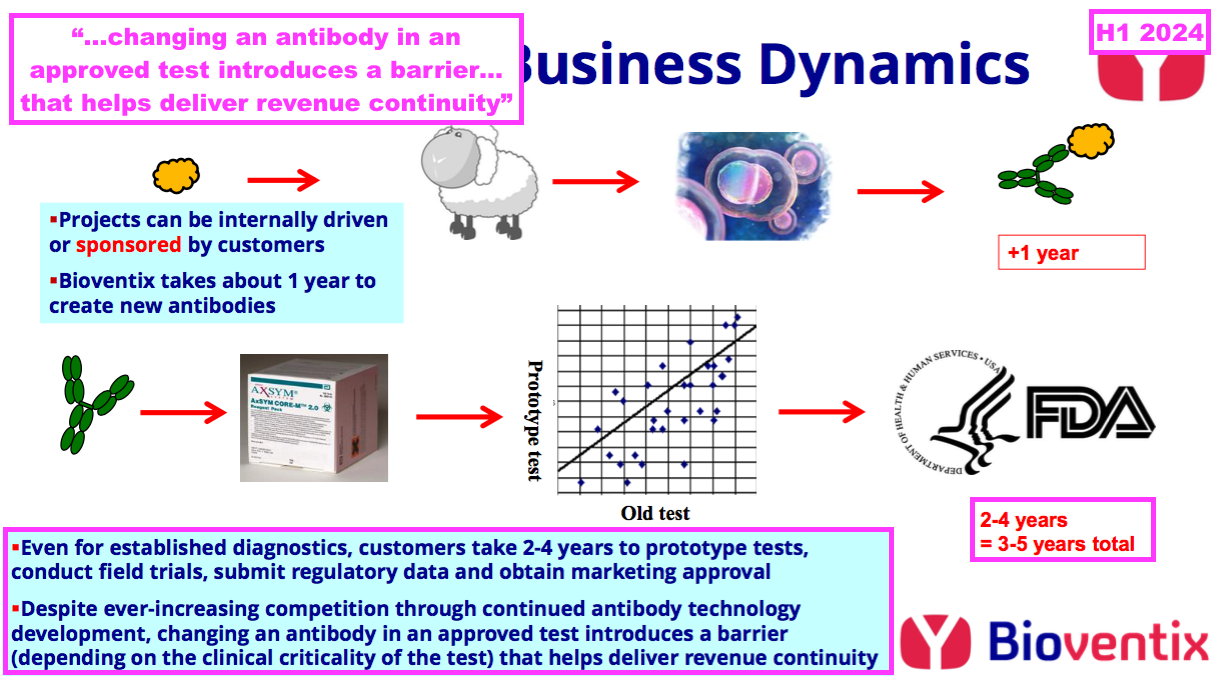
- As BVXP consistently declares within its annual reports, “there is a natural incentive for continued antibody use” because of the “time and cost to… validate the performance of [a] replacement antibody”:
[FY 2023] “There is a lead time of 4–10 years between our own research work and the receipt by Bioventix of royalty revenue from product sales. However, because of the resource required to gain such approvals, after having achieved approval for an accurate diagnostic test using a Bioventix antibody, there is a natural incentive for continued antibody use.
This results in a barrier to entry for potential replacement antibodies which would require at least partial repetition of the approval process arising on a change from one antibody to another. This barrier to antibody replacement arises from a combination of factors driven by the clinical criticality of the test and the potential consequences of making such a change which include the time and cost to register any changes required to validate the performance of the replacement antibody.”
Troponin and other antibodies
- This H1 admitted revenue from troponin antibodies — used within blood tests to help detect potential heart attacks — was “a little below expectation“:
“Whilst our total sales continue to grow, our sales relating to troponin antibodies were a little below expectation. We continue to believe that temporary operational issues experienced by our partner customers have slowed the rollout of their improved troponin assays. Whilst this has inhibited growth short term, there is no obvious reason to doubt the previous forecasts for future growth of troponin related revenues in the longer term.”
- The slower progress is not ideal when the preceding FY reemphasised BVXP’s near-term growth prospects were still dependent mostly on troponin:
[FY 2023] “Over the next few years, the continued commercial development of the new troponin assays and their roll-out by our customers will have the most significant influence on Bioventix sales.”
- Mind you, BVXP did say the troponin slowdown was “temporary” and revenue was only a “little” less than expected.
- For context, troponin revenue gained 30% to £1.6m during the preceding FY and supported 13% of group revenue:

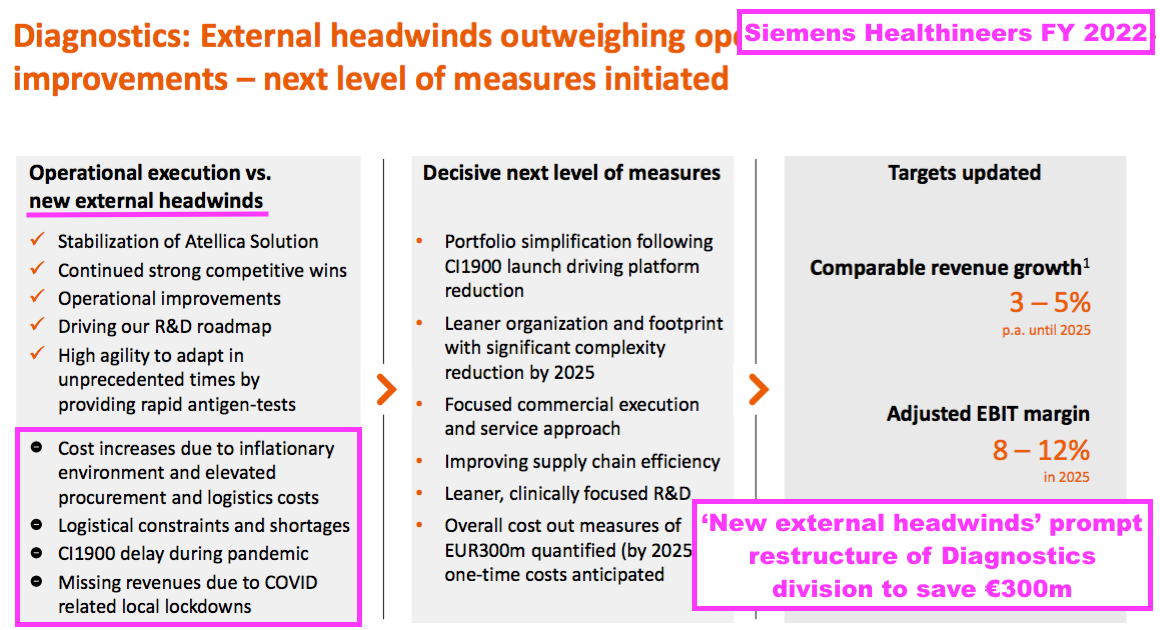
- I speculate BVXP’s reference to “operational issues experienced by our partner customers” refers to a divisional re-jig at primary troponin customer Siemens Healthineers.
- Siemens Healthineers announced a restructure of its Diagnostics division during 2022 to save €300m a year at a mooted cost of €300m-€450m:
- This H1 claimed there was “no obvious reason to doubt the previous forecasts for future growth of troponin-related revenues in the longer term”.
- I presume the “previous forecasts” were those contained in the broker note that accompanied the preceding FY:
[Cavendish October 2023] “Given the lower than forecast revenues in FY 2023 and H1, we have reduced our FY 2024 revenue forecast by £0.4m to £2.1m and introduced c.£2.8m in FY 2025E.
Peak revenues of troponin are still estimated to be in the £3m-3.5m range.“
- The broker had already reduced its troponin forecast by 16% to £2.1m for FY 2024, so this H1’s admission of slower troponin progress will presumably cut the projection once again.
- “Peak” troponin revenue of “£3m-£3.5m” by 2032 suggests a compound annual growth rate of approximately 8% from the preceding FY’s £1.6m.
- Annualised growth of 8% does not seem inspiring given troponin first registered sales during FY 2019…
- …and BVXP has said its much older antibodies can collectively advance at between 5% and 10%.
- Those older antibodies include biotin, which I understand has been sold since 1999…
- …and tri-iodothyronine (T3), which became commercial during 1995:
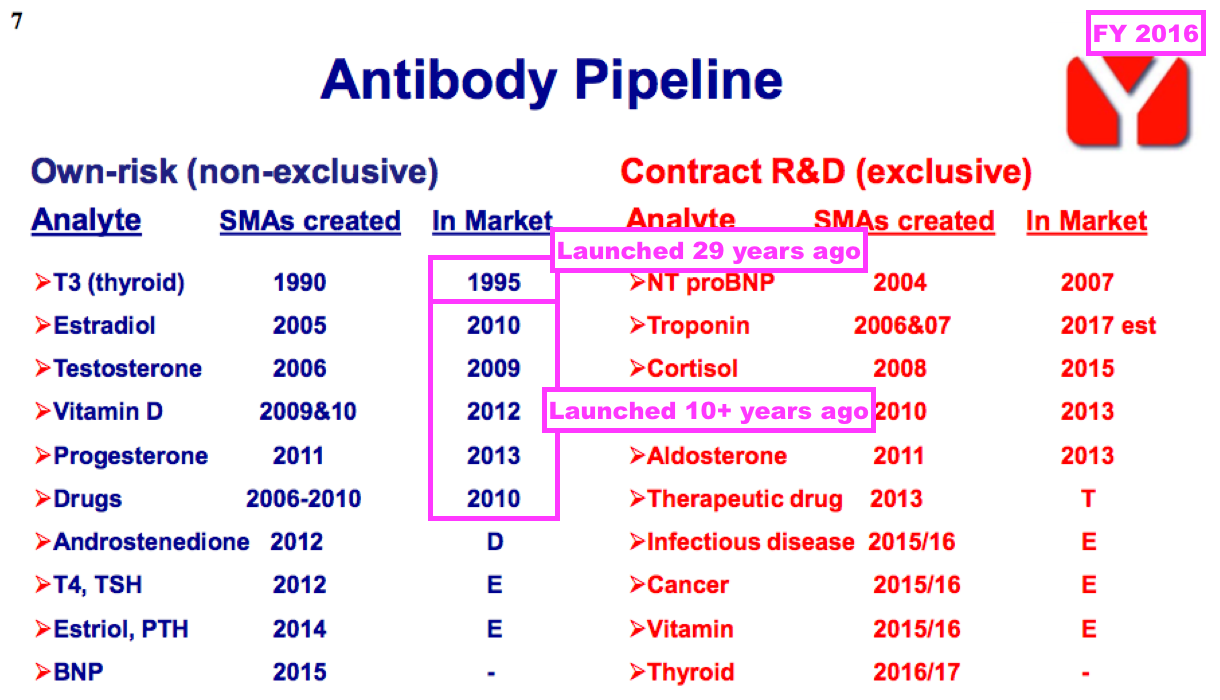
- The preceding FY was the first statement to confirm troponin revenue would cease during 2032. Development work on troponin commenced during 2006 under an exclusive usage contract with Siemens Healthineers.
Pipeline: Alzheimer’s and pTau217
- This FY provided further commentary about the R&D that one day might create a blood test that accurately predicts the likelihood of developing Alzheimer’s disease (AD).
- To recap, AD is characterised by the excessive accumulation of proteins — amyloid beta and tau — within and around brain cells. The accumulation typically commences years before the AD symptoms become obvious:
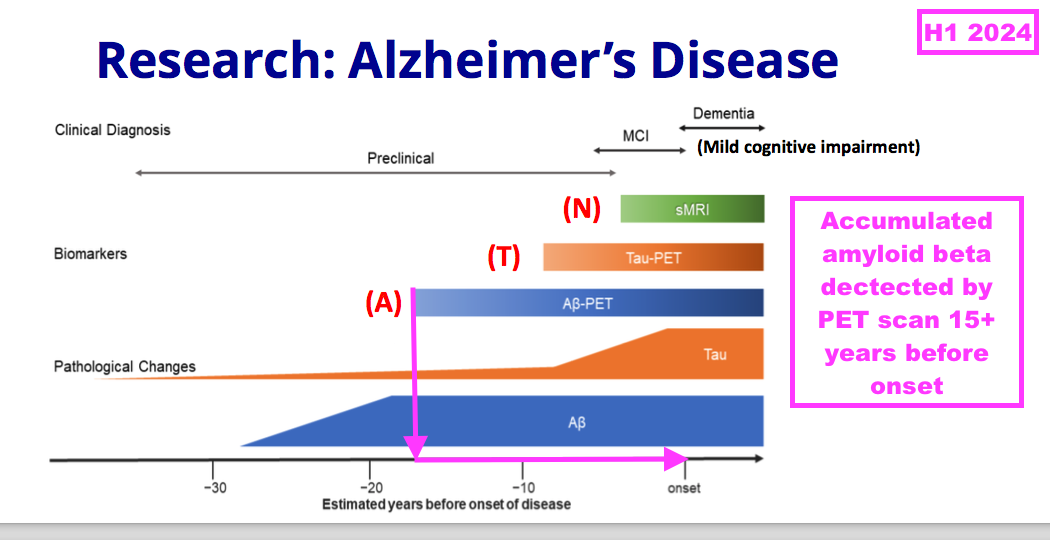
- AD is currently identified and/or monitored through brain scans and lumbar punctures, but the ambition among AD researchers is identification and/or monitoring through simple/fast/cheap blood tests using diagnostic antibodies.
- The recent emergence of treatments to slow the onset of AD — (Lecanemab from EISAI/Biogen and Donenamab from Eli Lilly) — has triggered a greater need to identify/monitor patients suitable for such treatments.
- Two years ago, BVXP announced an AD-research collaboration with the University of Gothenburg (UGOT) while also confirming its AD research had in fact commenced during 2020:
[H1 2022] “Since the summer of 2020, a considerable amount of our laboratory resources has been focused on the Tau biomarker which shows exciting potential in neuropathological diseases including Alzheimer’s. Some new antibodies were made during 2021 and we have more antibodies in the development pipeline for 2022.
The antibodies have been and will continue to be subjected to assay development and validation using clinical samples at the world-renowned laboratory of Kaj Blennow and Henrik Zetterberg at the University of Gothenburg. We are delighted with the continuing development of this collaboration and look forward to the generation of new data with our partners during 2022.”
- This H1 referred to ongoing AD testing by UGOT with a BVXP pTau217 prototype:
“Our research activities continue in line with the plans we described in our 2023 annual report. As detailed then, it is encouraging that after having invested a considerable amount of laboratory resource in the Tau project and Alzheimer’s disease (AD) diagnostics, our work with the University of Gothenburg (“UGOT”) has resulted in prototype assays for use in AD.
One prototype assay for early AD detection measures a leading candidate blood biomarker for early amyloid build-up which is, confusingly, a phosphorylated form of Tau called pTau217. This “UGOT” prototype assay performs well and in a similar way to pTau217 assays from other leading groups.”
- The preceding FY had directed shareholders to this UGOT scientific paper about pTau217: “A novel ultrasensitive assay for plasma p-tau217: performance in individuals with subjective cognitive decline and early Alzheimer’s disease“, Fernando Gonzalez-Ortiz, Journal of the Alzheimer’s Association, accepted for publication October 2023.
- My layman reading of that scientific paper led to three highlights:
- pTau217 is generally recognised as being the “most promising… p-tau biomarker” for identifying the likely onset of AD;
- Unlike other pTau217 studies, BVXP/UGOT’s pTau217 efforts helped identify “cognitively unimpaired” patients likely to develop AD, and;
- Unlike other pTau217 tests, the BVXP/UGOT pTau217 prototype came with no commercial restrictions.
- BVXP is one of many antibody developers that has worked on a pTau217 test for AD purposes. This H1 was the first statement in which BVXP name-checked a few of its rivals:
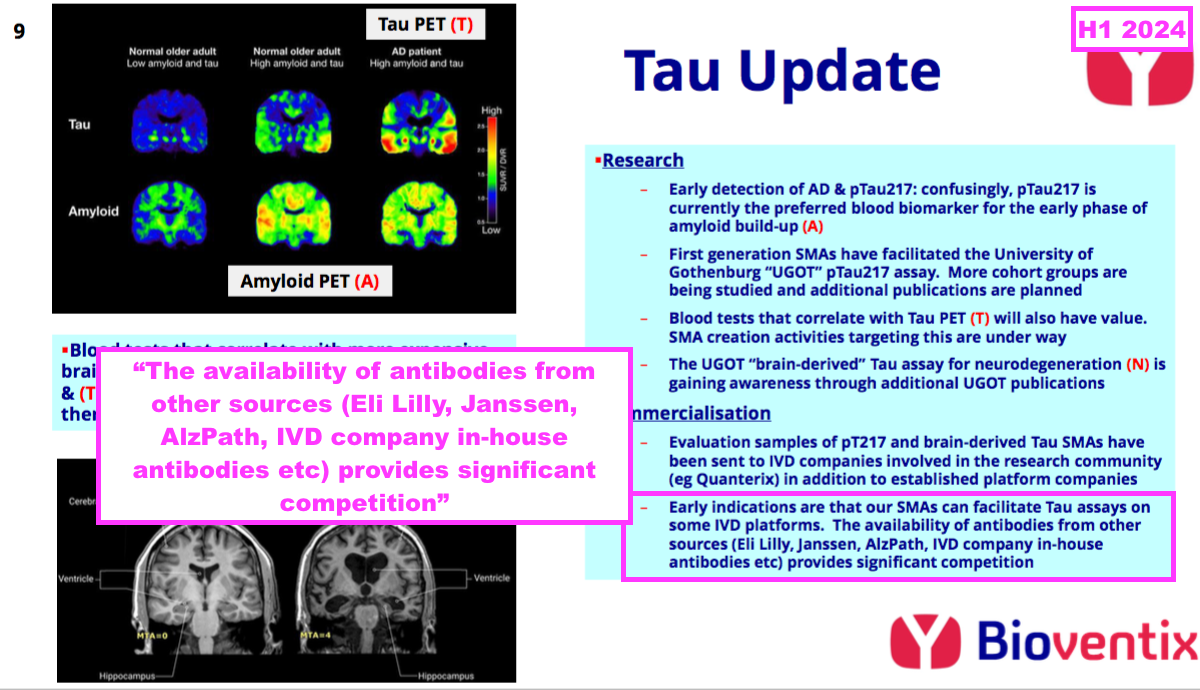
- pTau217 tests from ALZpath and Janssen are meanwhile available through Quanterix (see Pipeline: Alzheimer’s and brain-derived tau):

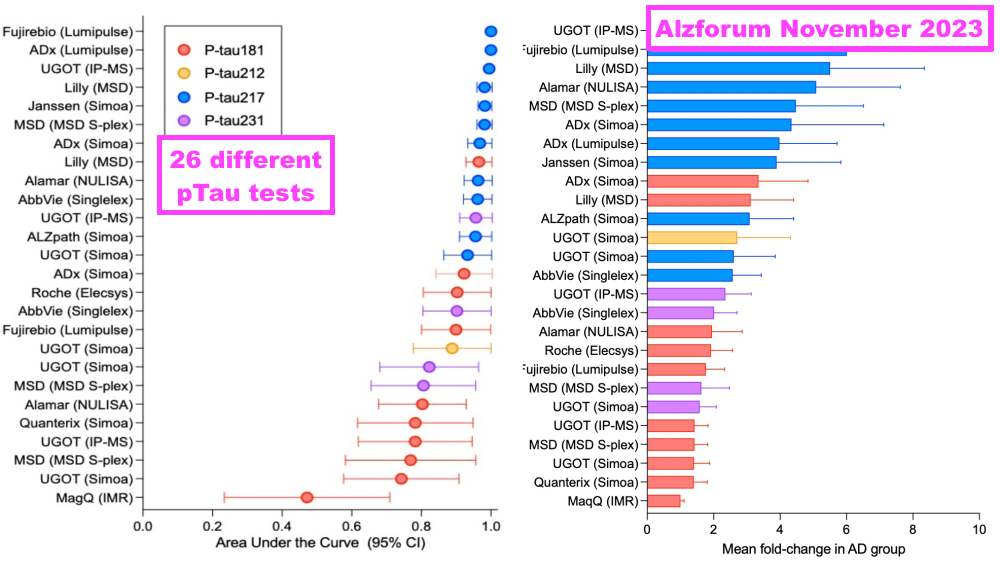
- Different antibody developers share the same AD experts.
- Although BVXP collaborates with the “world-renowned laboratory of Kaj Blennow and Henrik Zetterberg at the University of Gothenburg”…
- …the same laboratory collaborates with other antibody developers.
- ALZpath for instance also utilises the services of Kaj Blennow and Henrik Zetterberg:

- Mr Zetterberg has even compared pTau217 tests from C2N Diagnostics, Fujirebio, ALZpath, Janssen and Roche in a head-to-head study.
- In fact, the small-print within this BVXP-related scientific paper reels off a veritable Who’s Who of industry advisory connections to Messrs Blennow and Zetterberg:
[Nature March 2024] “[Henrik Zetterberg] has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alze-cure, Biogen and Roche.
[Kaj Blennow] has served as a consultant or at advisory boards for Abcam, Axon, BioArctic, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Ono Pharma, Pharmatrophix, Prothena, Roche Diagnostics and Siemens Healthineers.“
- Messrs Blennow and Zetterberg have co-founded their own biomarker business…
[Nature March 2024] “[Henrik Zetterberg] and [Kaj Blennow] are co- founders of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures- based platform company at the University of Gothenburg.“
- …which presumably adds a financial incentive to their academic/professional incentive to undertake as many AD studies as possible with as many antibody developers as possible.
- While pTau217 has seemingly become a crowded AD-research field, AD opportunities may lie within other tau epitopes.
- In particular, BVXP has worked on pTau212 and contributed to this scientific paper — “Plasma p-tau212 antemortem diagnostic performance and prediction of autopsy verification of Alzheimer’s disease neuropathology“ — published just before this H1 during March 2024.
- My layman reading of this scientific paper concluded pTau212 is very similar to pTau217 for detecting the excessive accumulation of amyloid beta and the possible early signs of AD.
- From what I can tell, the paper was the first to report specifically on the potential of pTau212 as an early AD indicator.
Pipeline: Alzheimer’s and brain-derived tau
- This H1 reported a second UGOT AD prototype had attracted “significant attention“:
“There is a second UGOT prototype assay for neuro-degeneration based on our antibodies that has received significant attention following recent publications in the scientific literature. We believe this assay could have utility in the monitoring of patients later in the disease and treatment pathway which will be a key requirement for assessing the efficacy of such new treatments.”
- I believe this second prototype relates to the following scientific paper — “Plasma brain-derived tau is an amyloid associated neuro-degeneration biomarker in Alzheimer’s disease” — that was published after this H1 during April 2024.
- My layman reading of this scientific paper derived the following points:
- A “substantial proportion” of individuals identified by a pTau217 test (or similar) as exhibiting an accumulation of amyloid beta do not actually develop AD;
- As per this earlier BVXP-related scientific paper, brain-derived tau within the blood is a promising signal of AD neuro-degeneration, and;
- Combining a test for accumulated amyloid beta (e.g. pTau217) with a test for brain-derived tau can identify the individuals at greater risk of significant, near-term “cognitive decline” through AD.
- The scientific paper’s usage of “hitherto elusive” suggests the findings were not confirmation of a previous study:
[Nature April 2024] “Plasma [brain-derived] tau provides insights into the hitherto elusive AD-type neuro-degeneration process in blood.“
- My online searching for ‘brain derived tau‘ refers only to BVXP and BVXP-related scientific papers, which suggests BVXP may have some sort of R&D edge with this particular AD project.
- I therefore speculate BVXP’s brain-derived tau antibody (TauJ.5H3) is more likely to become a commercial success than the company’s pTau217 test for accumulated amyloid beta.
- This H1 mentioned sending AD antibody samples to Quanterix (QTRX):
“After the scientific progress detailed in the our annual report, our focus has evolved towards refining the prototype tests described above and sending evaluation samples of SMAs to IVD companies that serve the research community, such as Quanterix, and our established global IVD customers. Early indications suggest that our SMAs could facilitate Tau assays on commercial platforms although we are aware that competing antibodies exist from other respected sources in addition to antibodies created in-house by our IVD company partners.”
- QTRX is a $475m Nasdaq-quoted manufacturer of “ultrasensitive” immunoassay analysers, which are apparently “up to 1000x” more powerful than conventional analysers:

- QTRX reckons its machines are the equipment of choice for AD research:
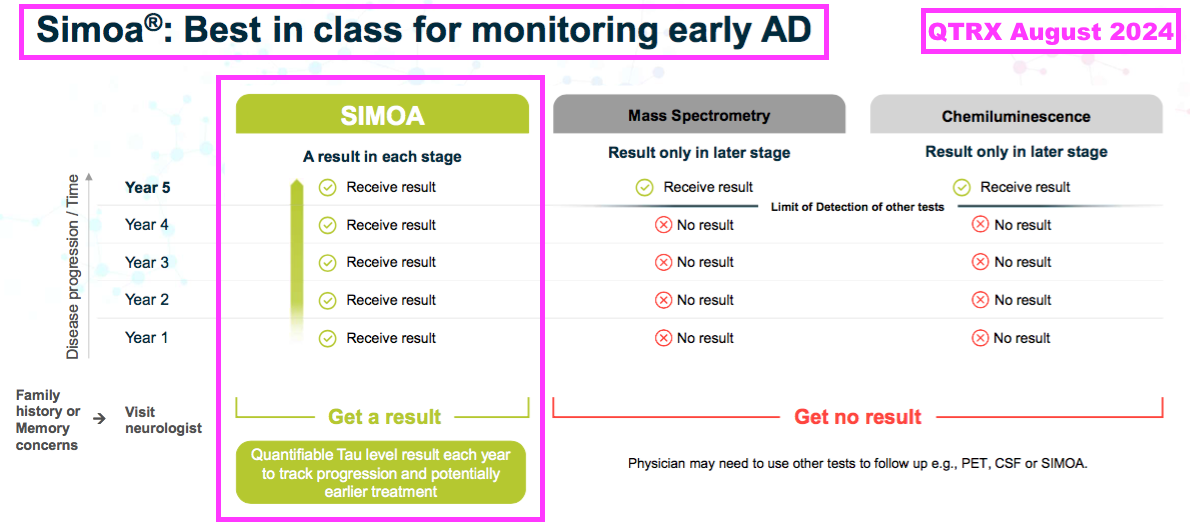
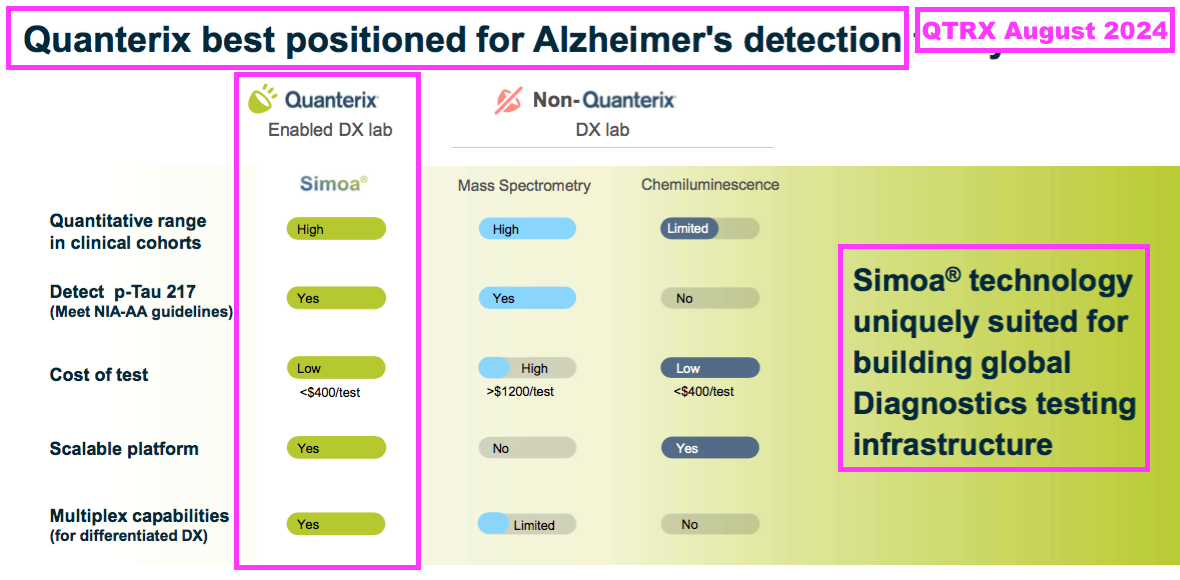
- QTRX highlights estimates of between $3b and $10b for the potential size of the AD diagnostics market:

- QTRX’s Q1 2024 results referred to BVXP’s brain-derived tau antibodies becoming available for use during Q2 2024:
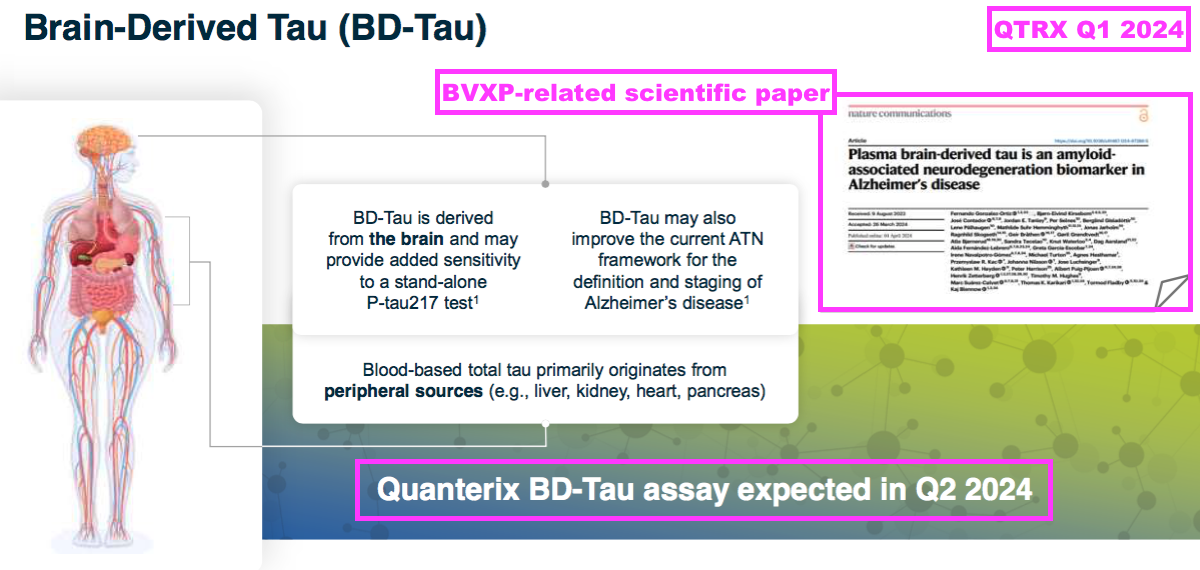
- Sure enough, BVXP’s brain-derived tau antibodies are currently available through QTRX for research purposes:
- The preceding FY had implied BVXP’s AD research could become commercial by 2028 at the earliest:
[FY 2023] “By the same dynamics, the current research work active at our laboratories now is more likely to generate sales in the period 2028–2038.“
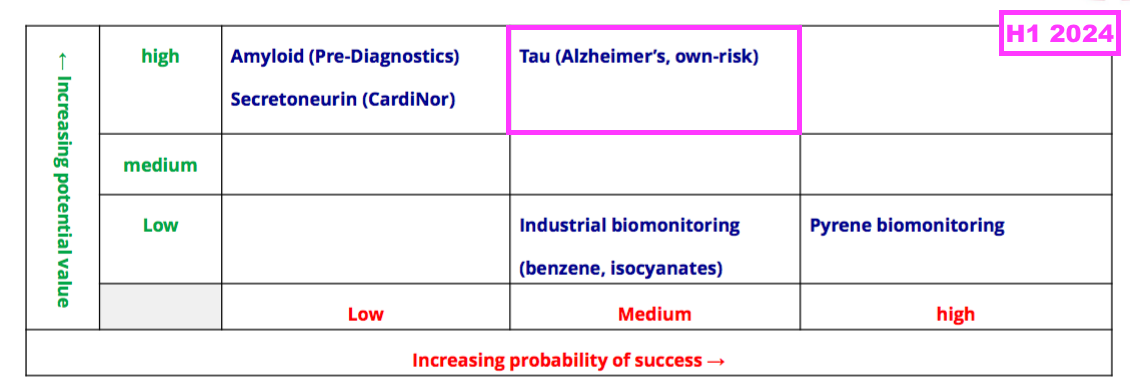
- The commercial potential of AD research remains High while the probability of AD-research success remains Medium:
- This H1 revealed the third part of BVXP’s AD research — creating antibodies that correlate with tau-tangle PET scans to identify more advanced cases of AD — has now commenced:
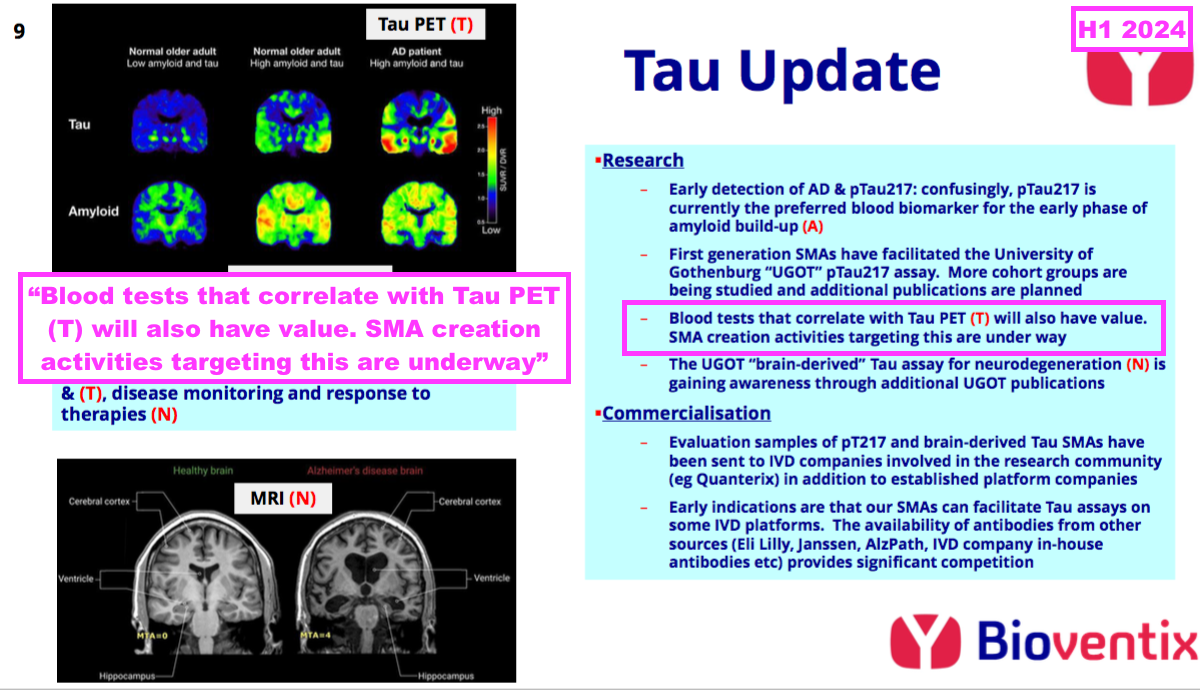
- I am not sure whether AD monetisation requires the full hat-trick of a working pTau217 amyloid-beta test, a working brain-derived tau test and a working ‘tau tangle’ PET-equivalent test.
Pipeline: biomonitoring
- BVXP’s pipeline grid continues to rate the company’s two biomonitoring projects as Low potential value:
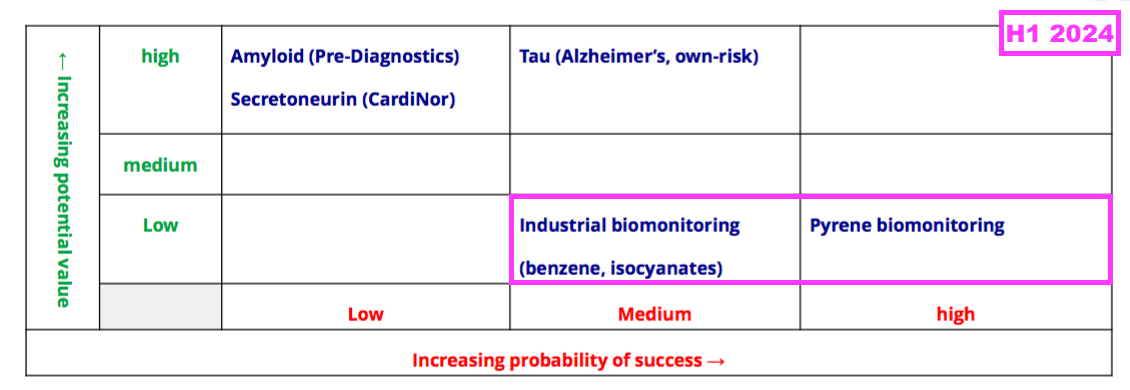
- The projects aim to identify particular industrial pollutants in a faster, easier and cheaper way than conventional tests, which involve sending all samples to central laboratories for expensive chromatography analysis.
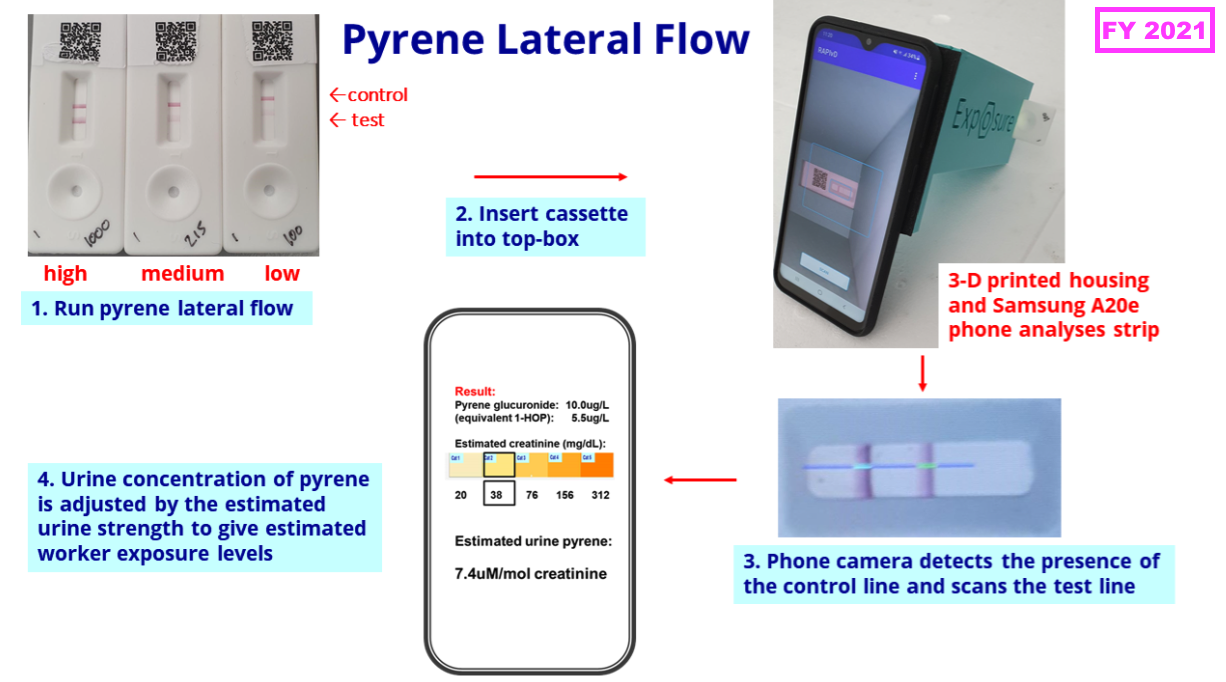
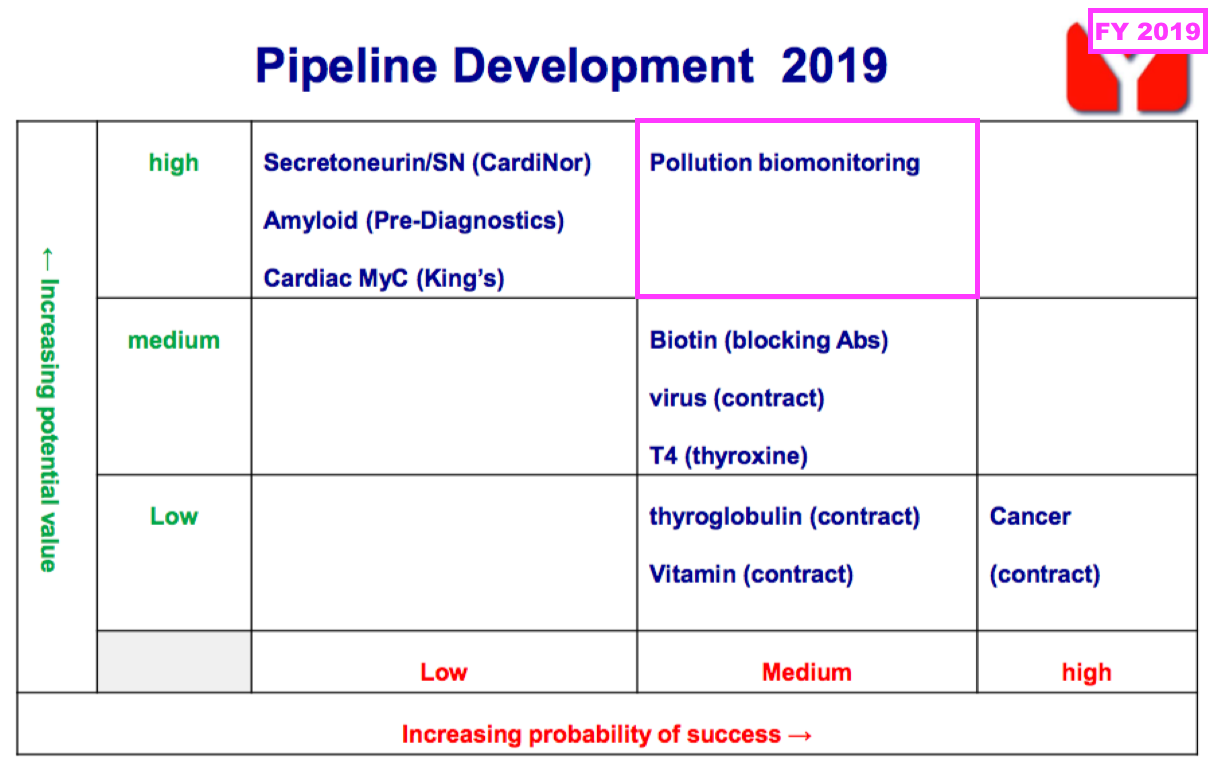
- Biomonitoring was first mentioned within the pipeline grid during FY 2019…
- …and this H1 revealed a third field trial is planned for the pyrene test:
“We are pleased with the continued development of our industrial pollution exposure assays. We are planning a field trial with firefighters using our prototype lateral flow test for pyrene, previously trialled in industrial workers’ urine. Antibody developments are in progress for two additional industrial pollutants, benzene, associated with the petrochemical industry, and isocyanates, used in the plastics and paints industry. We plan to continue our internal and external investment in these areas.“
- A scientific paper published after this H1 during October 2024 reported both the on-site and lab-based pyrene tests performed similarly to conventional chromatography when tested against a group of workers exposed to occupational pollutants…
- …although the on-site version created a number of ‘false positives’ due to the presence of creatinine in some samples.
- The scientific paper concluded the on-site version could be used as a ‘pre-screener’ to identify samples for further analysis that would reduce testing costs overall:
[Toxicology Letters October 2024] “Urinary analysis by [lateral flow] takes approximately 30 mins providing results in real-time. This enables the immediate confirmation of safe-working practice and provides immediate reassurance to workers involved in potentially hazardous tasks. Positive samples can be re-tested. Positive samples may also be sent for confirmatory analysis. Sending selected samples for laboratory analysis will greatly reduce the costs associated with packaging, transport and laboratory determination and enable a greater number of samples to be determined at more regular intervals. This should greatly increase the benefit and efficacy of biomonitoring.“
- The scientific paper also included a favourable snippet about the practicalities of the on-site test:
[Toxicology Letters October 2024] “Health professionals found the point of use test quick and easy to complete and the smartphone camera and app simple to use.”
Pipeline: CardiNor and Pre-Diagnostics
- Collaborations with Norwegian research houses CardiNor and Pre-Diagnostics remain stuck with a Low probability of success:
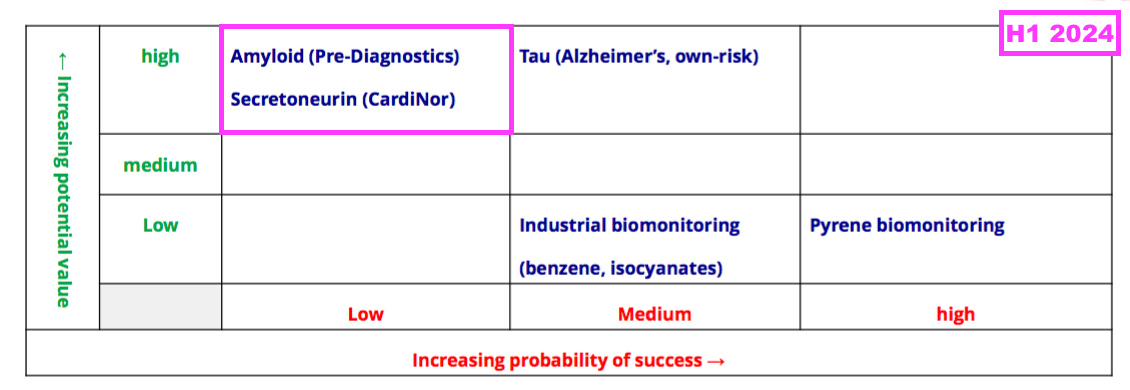
- BVXP invested an aggregate £610k into CardiNor and Pre-Diagnostics between FYs 2016 and 2020.
- The comparable H1 suggested the two Norwegian projects might issue news during 2023:
[H1 2023] “We continue to await news and critical data from both of our partners in Oslo; on our secretoneurin project with CardiNor for enhancing cardiac diagnostics and on our amyloid beta project with Pre-Diagnostics in Alzheimer’s diagnostics. We hope to have more news on these two projects during 2023.“
- But the preceding FY did not disclose any Norwegian progress:
[FY 2023] “Pre-Diagnostics (in Oslo) and their clinical collaborators have two amyloid beta assays based on Bioventix antibodies available for research use. A current focus for Pre-Diagnostics is ARIA (amyloid related imaging abnormality) which is an important side-effect of new anti-amyloid drugs for Alzheimer’s. Pre-Diagnostics assays relate to amyloid metabolism and could help screen for ARIA vulnerable patients, before or during treatment.
Our partners at CardiNor (also in Oslo) have continued with their work to try and identify the possible utility of secretoneurin in heart failure patients and in particular those patients who might be candidates for implantable cardiac devices (ICDs)”
- This H1 suggested Norwegian news may now emerge during 2024:
“We continue to await news and critical data from both of our partners in Oslo; on the secretoneurin project with CardiNor for enhancing cardiac diagnostics and on the amyloid beta project with Pre-Diagnostics in Alzheimer’s diagnostics. We hope to have more news on these two projects during 2024.“
- CardiNor’s secretoneurin project tests for a particular type of heart failure, with development work starting during 2015.
- CardiNor’s progress does not seem positive. The group last issued news during December 2023 when a fresh chief executive was appointed to bring “crucial competence“:
[CardiNor December 2023] “Bjørn Fuglaas, Chairman of the board, states; I am very pleased that Thomas [Grünfeld, the new chief executive] is strengthening the team, and he brings with him crucial competence at this stage of CardiNor’s development.“
- The new boss coincides with extra funding that, according to results from CardiNor shareholder Intuitive Investments (IIG), took place sometime during the six months to September 2023.
- IIG continues to value its CardiNor investment based on the “last investment round“:
[IIG H1 2024] “Investment of £122,000 in ordinary shares, held at £58,000 fair value, for which last investment round is deemed the most appropriate basis of measurement.”
- Despite carrying its investment at a 52% loss and not participating in the last investment round, IIG claims CardiNor has “made excellent progress“:
[IIG H1 2024]
“CardiNor has made excellent progress particularly with the amount of money raised, which includes:
• Elisa test CE marked with clear route to market in Europe and next generation magnetic test being developed.
• RuO in the US, but distribution deal done with IBL and talking to Labcorp. Going for full FDA approval.“
- The preceding FY and this H1 did not show BVXP participating in CardiNor’s latest funding.
- BVXP continues to carry its CardiNor investment at the original purchase value, which may be questionable given IIG has written down its investment.
- This 2018 document from CardiNor’s website indicates BVXP was then a 21.5% Cardinor shareholder.

- Pre-Diagnostics was formed during 2013 and its main focus is on developing tests that identify patients likely to suffer side effects from new AD treatments.
- Pre-Diagnostics appears more commercially optimistic than CardiNor. This August 2023 Pre-Diagnostics investment summary included remarks about a “licensing agreement by 2025, which also could create a basis for stock-listing or trade sale of shares“.
- That said, the same investment summary also implied sufficient cash to operate only until the end of 2025:
[Pre-Diagnostics August 2023] “The combination of grants and new equity would provide a financial runway until end of 2025“
- This 25-page Pre-Diagnostics investor presentation supplies further information, but the absence of any monetary values within the document is notable.
- This 2020 Pre-Diagnostics document indicates BVXP was at the time a 7.5% Pre-Diagnostics shareholder.
Financials: margin and employees
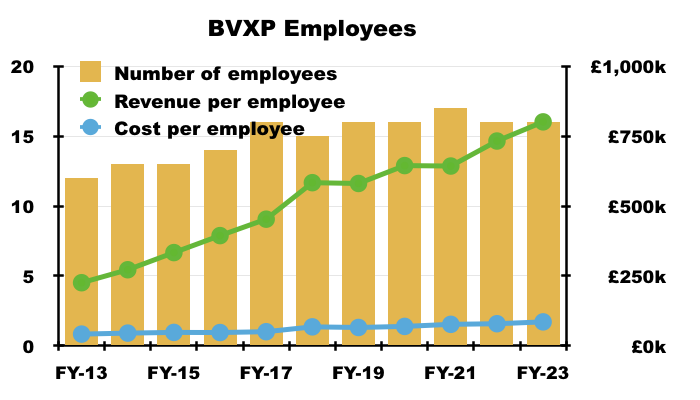
- Royalties earned from regular worldwide blood tests using antibodies developed by only 16 employees within a small laboratory continue to translate into some wonderful accounts.
- This H1 enjoyed a terrific 77% operating margin, the highest for an H1 since H1 2020 (80%):
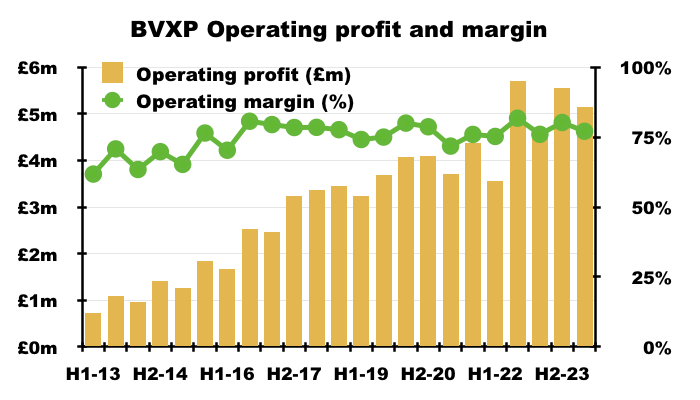
- BVXP’s margin has topped 70% since H2 2015.
- Cost of sales commendably advanced at a slower pace than revenue (5% versus 13%):
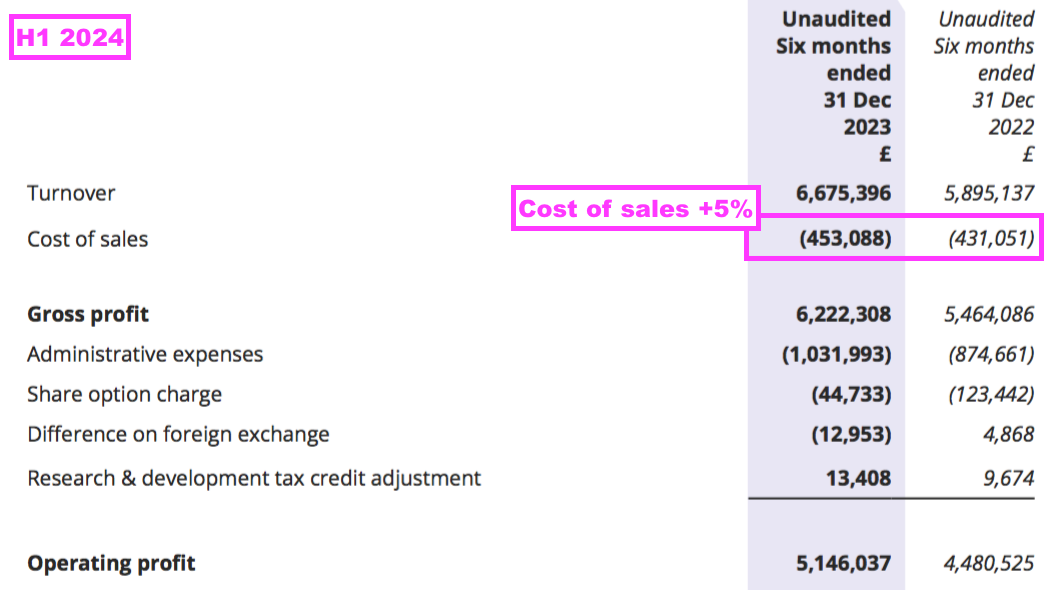
- Cost of sales includes the stock value of physical antibodies sold, and this H1’s increase was limited to £22k despite the greater physical sales to China.
- Administrative expenses advanced at a faster pace than revenue (18% versus 13%):
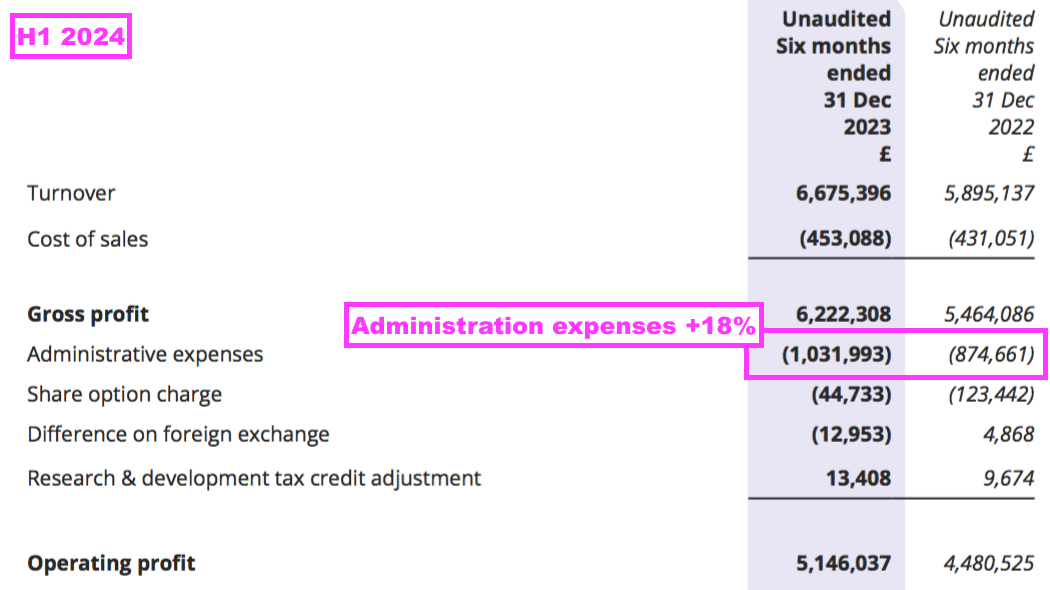
- BVXP’s largest expense is employee wages, which during the preceding FY advanced 14% versus revenue up 9%:

- I continue to wonder whether the 16-strong workforce will ever demand much greater salaries to reflect the significant economic value they have created for shareholders.
- Indeed, the huge £700k-plus gap between revenue per employee and cost per employee offers substantial headroom for higher pay:
- That said, the employees do seem very happy. The 2023 annual report (point 6) disclosed long average tenures:
[AR 2023] “Across the Company the average length of service at 30 September 2023 was 10 years 11 months, an increase of 5 months since 30 September 2022; our scientists have all been with the business for more than 5 years and our overall retention rate has been unchanged at 100% in each of the last 2 years.”
- A significant proportion of employee costs are classified as R&D, which is (commendably) all expensed as incurred and (presumably) generates no material revenue.
- Replacing “manual and spreadsheet-based documentation” (point 6) may even create more time for extra R&D:
[AR 2023] “During 2023 the team considered, reviewed and selected a cloud-based solution to automate the recording, management and communication of quality, production, research and development and operational processes replacing previously manual and spreadsheet-based documentation.”
Financials: cash flow and balance sheet
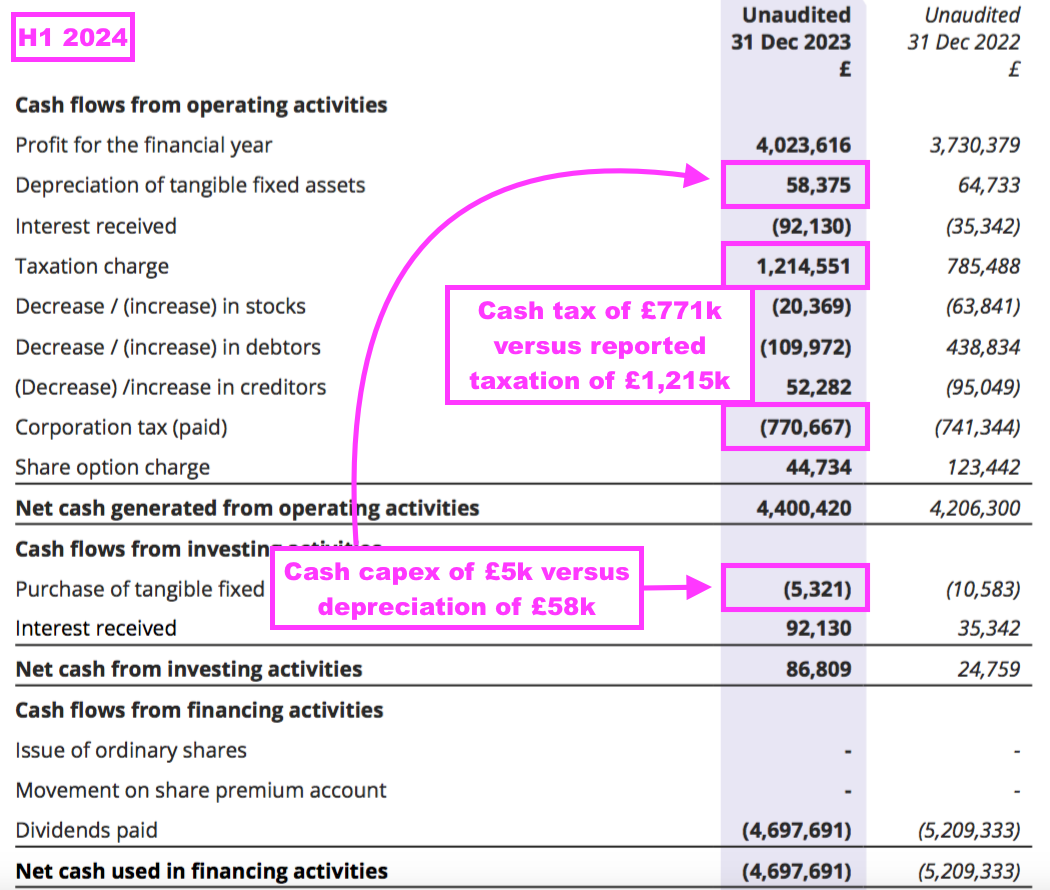
- BVXP’s capex during this H1 amounted to just £5k versus depreciation of £58k, and both figures were tiny versus earnings of £4m:
- The 2023 annual report (point 16) implied the company’s lab equipment had a useful life of only four years:
[AR 2023] “Land is not depreciated. Depreciation on other assets is charged so as to allocate the cost of assets less their residual value over their estimated useful life:
• Freehold property – 2% straight line
• Plant and equipment – 25% straight line
• Motor vehicles – 25% straight line
• Fixtures & Fittings – 25% straight line
• Office Equipment – 25% straight line“
- This H1 changed the depreciation rate to an effective useful life of more than six years:
“Tangible fixed assets are stated at cost less depreciation. Depreciation is not charged on freehold land. Depreciation on other tangible fixed assets is provided at rates calculated to write off the cost of those assets, less their estimated residual value, over their expected useful lives on the following bases:
• Freehold property – 2% straight line
• Plant and equipment – 15% straight line
• Motor Vehicles – 25% straight line
• Fixtures and Fittings – 15% straight line
• Office Equipment – 25% straight line”
- Expenditure on replacement equipment should therefore now occur a couple of years later than previously expected.
- The tiny capex plus a favourable £0.4m difference between reported tax and cash tax allowed earnings of £4.0m to convert into free cash of £4.5m, which in turn helped fund the preceding FY’s H2 dividend of £4.7m.
- BVXP’s cash position therefore finished the six months £0.2m lighter at £5.5m.
- The £5.5m cash position is not troubled by significant liabilities; the balance sheet carries no bank debt and no pension complications.
- The preceding FY reiterated the conservative stance towards the balance sheet:
[FY 2023] “Our view continues to be that maintaining a cash balance of approximately £5 million is sufficient to facilitate operational and strategic agility both with respect to possible corporate or technological opportunities that might arise in the foreseeable future.”
- BVXP has reported net cash of at least £5m since FY 2016:
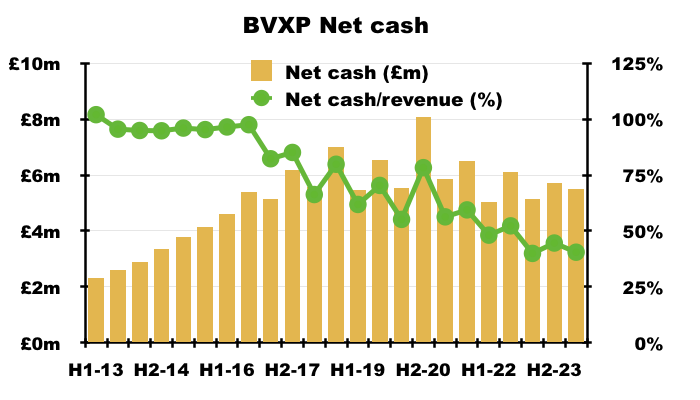
- This H1’s £5.5m net cash equates to 40% of trailing twelve-month revenue, a historically low level for BVXP but very high when compared to other portfolio members such as Tristel (27%), Andrews Sykes (25%) and FW Thorpe (18%).
- Bank interest amounted to £92k or a reasonable 3.3% on this H1’s average £5.6m cash position. 3.3% compares to an estimated 2.5% earned during the preceding H2 and an estimated 1.2% earned during the comparable H1.
- BVXP’s balance sheet still implies the business remains an inherently capital-light operation.
- Net assets of £11.4m less cash of £5.5m leaves £5.9m, represented by debtors (£5.9m), stock (£0.6m), tangible assets (£0.5m), the Norwegian investments (£0.6m) and creditors (£1.7m):
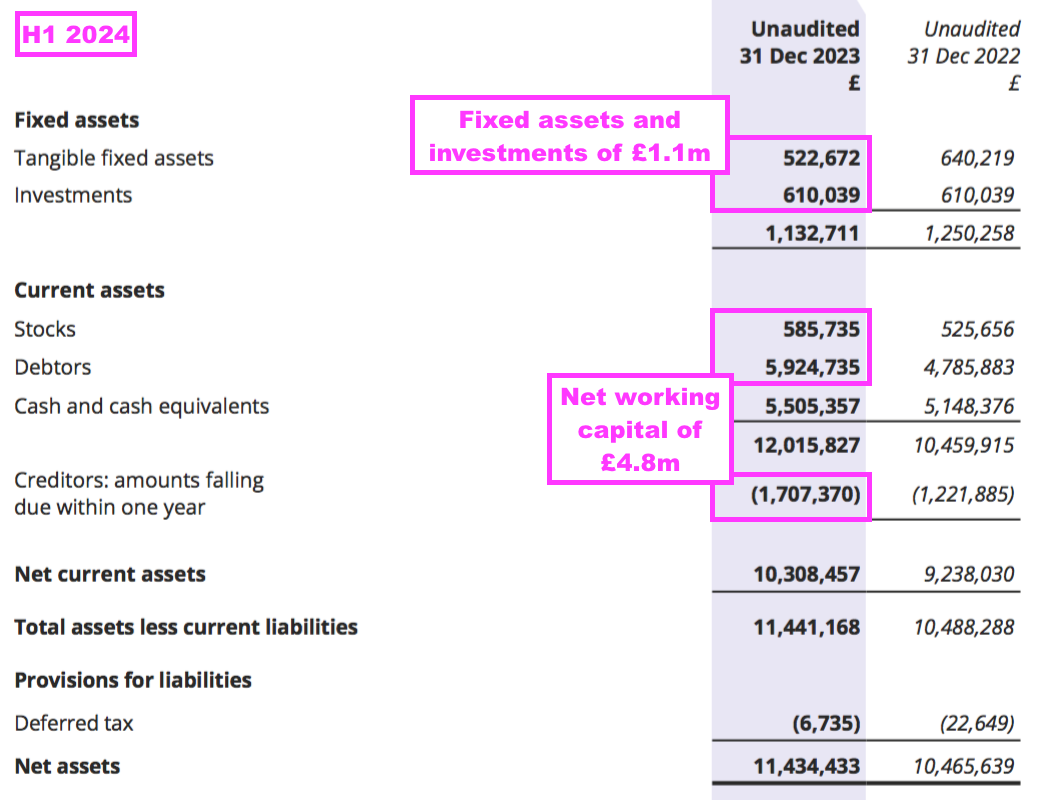
- Debtors mostly reflect ‘accrued income’, which are royalties recognised as revenue but have not been invoiced to the customer at the balance sheet date. By the time BVXP publishes its accounts, the royalties have been paid and the accrued income has therefore already been converted into cash.
- The £5.9m of aggregate debtors, stock, tangible assets, investments and creditors compares to trailing twelve-month earnings of almost £8.7m, and confirms BVXP can operate very profitably without retaining significant capital within the business.
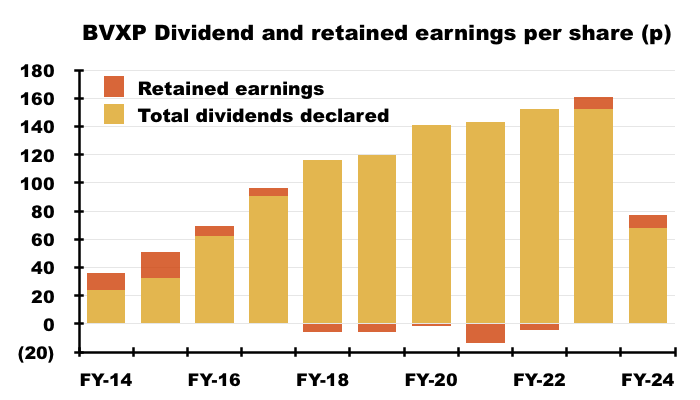
- Since FY 2014, BVXP has in fact declared aggregate earnings of £11.30 per share, of which £11.02 were distributed as ordinary or special dividends and the remaining 28p per share retained within the business:
Valuation
- This H1 supplied a positive outlook for H2 2024:
“In conclusion, our core business has performed in line with expectations with growth in China being a key feature. Troponin revenues did not accelerate quite as expected but we continue to believe that the headwinds are temporary and operational in nature. We remain excited as the scientific output of our Gothenburg Alzheimer’s collaboration slowly translates into commercial potential. We look forward to further progress in the second half of the year and beyond.”
- The positive H2 outlook was supported by the aforementioned broker projections:
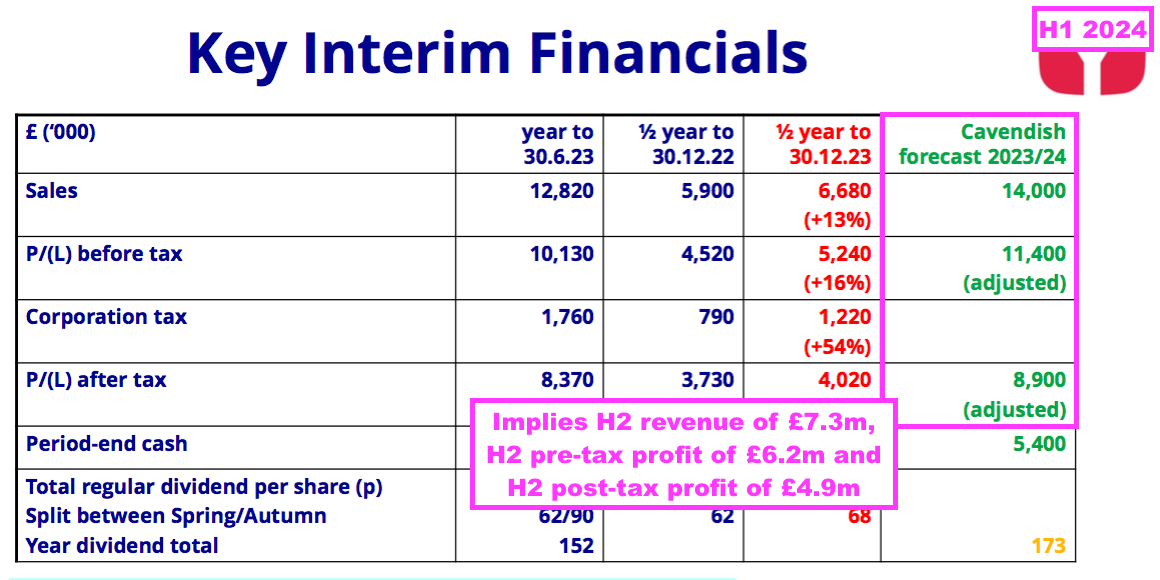
- Predicted FY 2024 revenue of £14.0m implies H2 2024 revenue of £7.3m — 6% higher than the £6.9m reported for the preceding H2.
- Predicted FY 2024 pre-tax profit of £11.4m implies H2 2024 pre-tax profit of £6.2m — 10% higher than the £5.6m reported for the preceding H2.
- Predicted FY 2024 post-tax profit of £8.9m implies H2 2024 post-tax profit of £4.9m — 14% higher than the £4.4m reported for the preceding H2.
- Predicted FY 2024 post-tax profit of £8.9m equates to 171p per share and supports a possible 22x multiple with the share price at £37.
- If the 171p per share earnings forecast is achieved, the 22x multiple would be towards the lower end of the range witnessed since 2017:
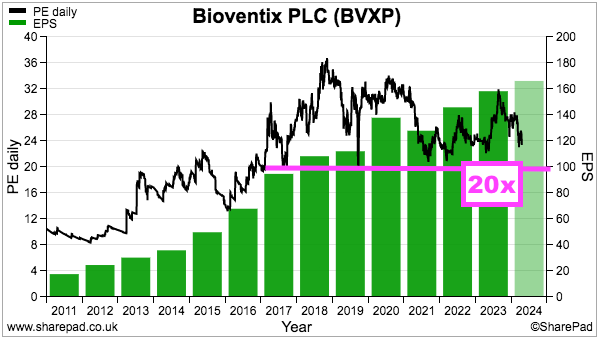
- I purchased BVXP during 2016 when the shares were valued at a c17x P/E multiple.
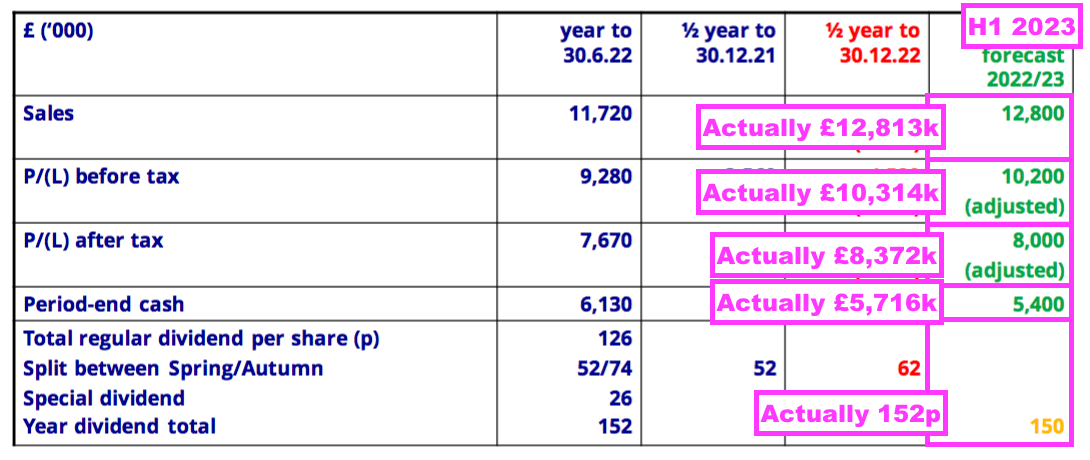
- BVXP’s broker was reasonably accurate with its FY 2023 predictions published within the comparable H1:
- Bear in mind troponin revenue will cease during 2032 and the associated profit should not therefore be valued on a simple P/E multiple.
- Assuming troponin revenue:
- Averages (a possibly ambitious) £2.5m for the next 8 years before expiry, and;
- Has no associated costs…
- …gives a value for troponin of £15m after 25% standard UK tax but before any time-value discounting.
- The table below derives the possible earnings from BVXP’s vitamin D and other commercialised antibodies by:
- Estimating calendar-year 2023 revenue without the (assumed unchanged) troponin income;
- Then subtracting all cost of sales and administration expenses;
- Then adding back (estimated) R&D costs, and;
- Then subtracting 25% standard UK tax:
| Estimated CY 2023 for vitamin D and other antibodies | (£k) |
| Revenue | 13,596 |
| Less (estimated) troponin revenue | (1,610) |
| Less cost of sales | (850) |
| Less admin expenses | (2,047) |
| Add back (estimated) R&D expense | 1,228 |
| Operating profit | 10,317 |
| Less tax at 25% | (2,579) |
| Earnings | 7,738 |
- Assuming vitamin D and the other commercial antibodies are a predictable source of income that could grow at 5% per annum, maybe their estimated earnings of £7.7m translate into a £155m ‘fair’ valuation if buyers were satisfied with an initial 5% cash yield.
- BVXP’s present £193m market cap less the £15m troponin estimate less the £155m vitamin D/other estimate leaves the pipeline valued at £23m.
- True, these valuation sums could be fine-tuned to:
- Calculate a more realistic net present value for troponin;
- Include R&D tax credits and other tax adjustments;
- Allocate cost of sales to the appropriate antibody;
- Incorporate the cash position and interest received, and;
- Apply different multiples to that £7.7m vitamin D/other earnings estimate.
- But valuing the pipeline at £23m may already seem optimistic given:
- Material pipeline upside probably rests entirely on AD;
- AD commercialisation is not expected until 2028 at the very earliest;
- Significant AD revenue may then take several years to emerge, and;
- R&D cash burn runs at approximately £1.2m per annum.
- That said, the pipeline ought to have some value to reflect the chance the AD efforts will one day become commercial.
- After all, the vitamin D antibody supports almost half of BVXP’s revenue… and may therefore be inherently worth almost half of BVXP’s £193m market cap….
- …and I speculate a commercially popular AD antibody would be more valuable than a commercially popular vitamin D antibody.
- BVXP’s broker has published calculations that show a successful pTau217 antibody could earn yearly royalties of approximately $7.5m throughout the 2030s:
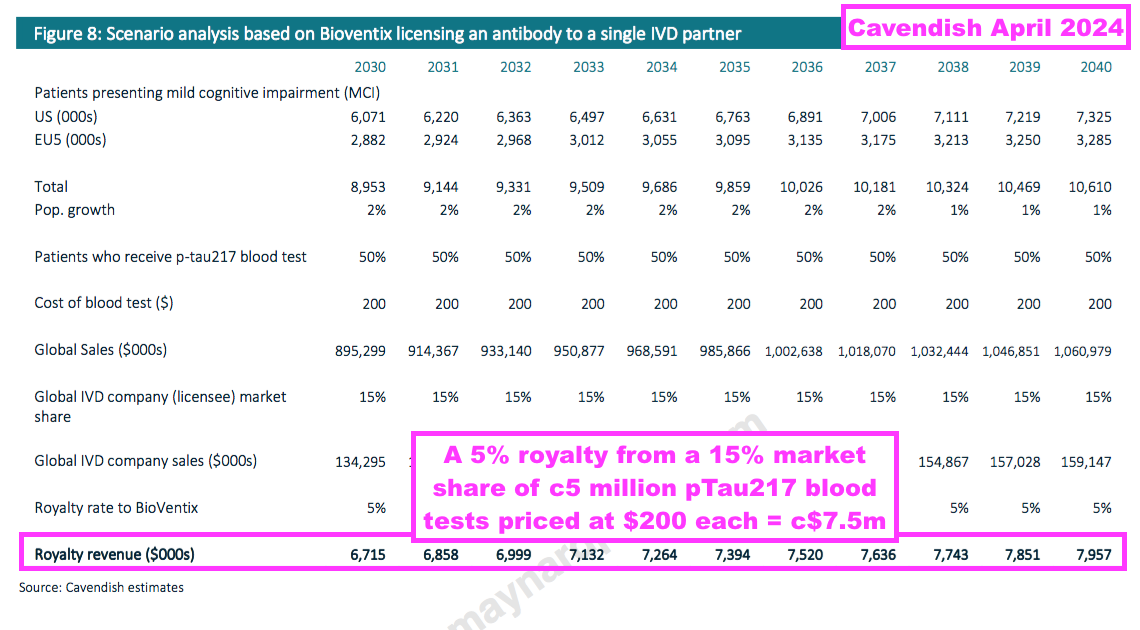
- Another valuation viewpoint may come from C2N Diagnostics, which has been awarded up to $50m (c£38m) since 2020 to develop a range of AD-related blood tests. Such an award might place a value on BVXP’s AD efforts.
- As well as the AD potential, core shareholder attractions include:
- The steady income from a stable of antibodies for ten years or more, due in part to the cost/time/upheaval/risk of replacing proven blood tests with upgraded versions;
- A competitive ‘moat’, helped in part by protracted development/regulatory timescales and ‘captive’ end-customers (e.g. hospitals) restricted to particular blood-test machines (and therefore particular diagnostic antibodies), and;
- The amazing margins and minimal reinvestment requirements, which underline the terrific royalty economics of antibodies if they become commercial.
- For now, longer-term upside seems almost entirely dependent on the success of the AD research to generate a step-change to future earnings and revitalise the range-bound share price:
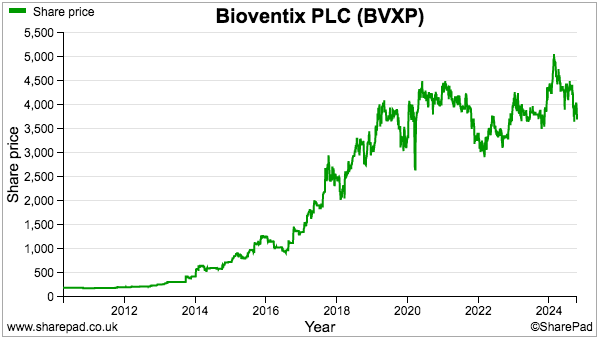
- While further AD developments are awaited, the trailing 158p per share dividend currently supplies a handy 4.3% income at £37.
Maynard Paton